Effect of Roflumilast Cream (ARQ-151) on Itch and Itch-Related Sleep Loss in Adults with Chronic Plaque Psoriasis: Patient-Reported Itch Outcomes of a Phase 2b Trial
- PMID: 36370336
- PMCID: PMC9968264
- DOI: 10.1007/s40257-022-00739-3
Effect of Roflumilast Cream (ARQ-151) on Itch and Itch-Related Sleep Loss in Adults with Chronic Plaque Psoriasis: Patient-Reported Itch Outcomes of a Phase 2b Trial
Abstract
Background: Itch is the most bothersome symptom reported by patients with psoriasis. Safe and effective treatments for psoriasis that also address itch are needed.
Objectives: To report effects of roflumilast cream on itch-related outcomes from a Phase 2b trial.
Methods: Adults with chronic plaque psoriasis were randomized to roflumilast 0.3%, roflumilast 0.15%, or vehicle once-daily for 12 weeks. Psoriasis severity was assessed via the Investigator Global Assessment (IGA; a 5-point scale assessing plaque thickening, scaling, and erythema ranging from 0 [clear] to 4 [severe]) and ≥ 2 on a modified Psoriasis Area and Severity Index (PASI-HD, which combines severity of lesions and area affected, ranging from 0 [no disease] to 72 [maximal disease], with the actual percentage of the anatomical area involved in those patients with < 10% of anatomical area involved [e.g., 0.1 for 1% to 0.9 for 9%]). Itch was evaluated via Worst Itch Numeric Rating Scale (WI-NRS), Psoriasis Symptom Diary (PSD) Items 1 (severity of itch) and 2 (bother of itch), and itch-related sleep loss NRS scores. Post hoc correlation analyses between WI-NRS and PASI, WI-NRS and itch-related sleep loss, and WI-NRS and DLQI were also performed.
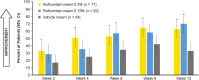
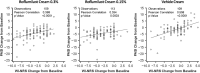
Results: Roflumilast-treated patients had significantly greater improvements than vehicle-treated patients in WI-NRS and PSD Items 1 and 2 beginning at Week 2 and in itch-related sleep loss Weeks 6 through 12. Among patients with baseline WI-NRS ≥ 6, significantly more patients achieved ≥ 4-point improvement with roflumilast than with vehicle as early as Week 2. Itch severity had low correlation with PASI while WI-NRS and IGA were not always aligned.
Limitations: The first assessment was at 2 weeks, limiting the ability to assess early onset of itch response.
Conclusion: Roflumilast cream improved itch and itch-related sleep loss associated with chronic plaque psoriasis.
Gov identifier: NCT03638258.
© 2022. The Author(s).
Conflict of interest statement
L. Stein Gold is an investigator for AbbVie, Arcutis, Amgen, Dermavant, Eli Lilly and Company, Leo, Novartis, Ortho Derm, and Pfizer; serves as an advisor for Amgen, Arcutis, BMS, Dermavant, Leo, Novartis, Ortho Derm, Pfizer, and UCB, is a speaker for Amgen, Leo, Ortho Derm, and Pfizer. J. Alonso-Llamazares is an investigator for Arcutis; speaker for Celgene (Amgen), Dermira (Eli Lilly), Eli Lilly and Company, Ortho Derm, and UCB Pharma; and serves on advisory boards for Leo. Z.D. Draelos received grant support from Arcutis Biotherapeutics, Inc. for the conduct of this study. M.J. Gooderham has been a speaker, advisory board member, investigator and/or consultant for AbbVie, Akros, Amgen, Arcutis, BMS, Boehringer-Ingelheim, Celgene, Dermavant, Dermira, Eli Lilly and Company, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Medimmune, Merck, Novartis, Pfizer, Regeneron, Sanofi Genzyme, Sun Pharma, UCB Pharma, and Valeant/Bausch. S.E. Kempers is an investigator for Arcutis Biotherapeutics, Inc., and serves as a consultant for Foamix and Kinex. L.H. Kircik is an investigator, consultant, speaker, and/or advisory board member for Abbott Laboratories, Acambis, Aclaris, Allergan, Inc., Almirall, Amgen Inc., Anacor Pharmaceuticals, Assos Pharma, Astellas Pharma US, Inc., Asubio, Berlex Laboratories (Bayer HealthCare Pharmaceuticals), Biogen-Idec, Biolife, Biopelle, Boehringer-Ingelheim, Breckinridge Pharma, Celgene, Cellceutix, Centocor, Inc., Cipher, Coherus, Colbar, CollaGenex, Combinatrix, Connetics Corporation, Coria, Dermavant, Dermik Laboratories, Dermira, Dow Pharmaceutical Sciences, Inc., Dusa, Eli Lilly and Company, Embil Pharmaceuticals, EOS, Exeltis, Ferndale Laboratories, Inc., Foamix, Genentech, Inc., GlaxoSmithKline, PLC, Health Point, LTD, Idera, Innocutis, Innovail, Intendis, Isdin, Johnson & Johnson, Laboratory Skin Care Inc., Leo, L'Oreal, 3M, Maruho, Medical International Technologies, Medicis Pharmaceutical Corp., Merck, Merz, Nano Bio, Novartis AG, Noven Pharmaceuticals, Nucryst Pharmaceuticals Corp., Obagi, Onset, OrthoNeutrogena, PediaPharma, PharmaDerm, Pfizer, Promius, PuraCap, QLT, Inc., Quinnova, Quatrix, Serono (Merck Serono International SA), SkinMedica, Inc., Stiefel Laboratories, Inc., Sun Pharma, Taro, TolerRx, Triax, UCB Pharma, Valeant Pharmaceuticals Intl, Warner-Chilcott, XenoPort, and ZAGE. M.G. Lebwohl reports receipt of research funds from AbbVie, Amgen, Arcutis, Boehringer-Ingelheim, Dermavant, Eli Lilly and Company, Incyte, Janssen Research & Development, LLC, Leo Pharma, Ortho Dermatologics, Pfizer, and UCB Pharma and serves as a consultant for Aditum Bio, Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., BMD Skincare, Boehringer-Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Facilitate International Dermatologic Education, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Serono, Theravance, and Verrica. K.A. Papp is an investigator, consultant, speaker, scientific officer or has served on steering committees or advisory boards for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer-Ingelheim, Bristol Myers Squibb, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dice Pharmaceuticals, Dow Pharma, Eli Lilly and Company, Evelo, Galapagos, Galderma, Gilead, GSK, Incyte, Janssen, Kyowa Hakko Kirin, Leo, Medimmune, Meiji Seika Pharma, Merck (MSD), Merck Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, Takeda, UCB, and Xencor. D.M. Pariser is an investigator, consultant, and/or advisory board member for Abbott Laboratories, Almirall, Amgen, AOBiome, LLC, Asana Biosciences, LLC, Atacama Therapeutics, Bickel Biotechnology, Biofrontera AG, BMS, Celgene Corporation, Dermavant Sciences, Dermira, Eli Lilly and Company, LEO Pharma, US, Menlo Therapeutics, Merck & Co., Inc, Novartis Pharmaceuticals Corp., Novo Nordisk A/S, Ortho Dermatologics, Pfizer Inc., Regeneron, Sanofi, Stiefel, a GSK company, TDM SurgiTech, Inc., TheraVida, and Valeant Pharmaceuticals International. D.P. Toth is an investigator and/or consultant for AbbVie, Amgen, Arcutis, Avillion, Bausch Health/Valeant, Bristol Myers Squibb, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly and Company, Galderma, Genentech, GSK, Incyte, Janssen, Leo Pharma, Merck Serono, Medimmune, Novartis, Pfizer, Regeneron, Roche, Sanofi-Aventis/Genzyme, Sun Pharma, and UCB Pharma. G. Yosipovitch has received grant/research support from Bellus Health, Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc., and Sanofi Regeneron and has been a consultant for Bellus Health, Eli Lilly and Company, Galderma, Kiniksa, Leo Pharma, Novartis, Pfizer Inc., Sanofi Regeneron, and Trevi. R. Higham, A. Feng, and D.R. Berk are employees of Arcutis Biotherapeutics, Inc.
Figures



References
-
- World Health Organization . Global report on psoriasis. Geneva: World Health Organization; 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

