An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study
- PMID: 36370413
- PMCID: PMC10099462
- DOI: 10.1002/jbm.b.35186
An in vitro comparison of human corneal epithelial cell activity and inflammatory response on differently designed ocular amniotic membranes and a clinical case study
Abstract
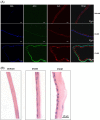
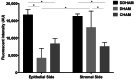
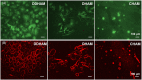
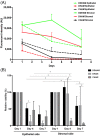
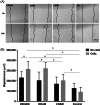
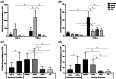
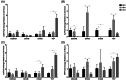
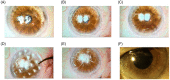
Amniotic membrane (AM) is a naturally derived biomaterial with biological and mechanical properties important to Ophthalmology. The epithelial side of the AM promotes epithelialization, while the stromal side regulates inflammation. However, not all AMs are equal. AMs undergo different processing with resultant changes in cellular content and structure. This study evaluates the effects of sidedness and processing on human corneal epithelial cell (HCEC) activity, the effect of processing on HCEC inflammatory response, and then a case study is presented. Three differently processed, commercially available ocular AMs were selected: (1) Biovance®3L Ocular, a decellularized, dehydrated human AM (DDHAM), (2) AMBIO2®, a dehydrated human AM (DHAM), and (3) AmnioGraft®, a cryopreserved human AM (CHAM). HCECs were seeded onto the AMs and incubated for 1, 4 and 7 days. Cell adhesion and viability were evaluated using alamarBlue assay. HCEC migration was evaluated using a scratch wound assay. An inflammatory response was induced by TNF-α treatment. The effect of AM on the expression of pro-inflammatory genes in HCECs was compared using quantitative polymerase chain reaction (qPCR). Staining confirmed complete decellularization and the absence of nuclei in DDHAM. HCEC activity was best supported on the stromal side of DDHAM. Under inflammatory stimulation, DDHAM promoted a higher initial inflammatory response with a declining trend across time. Clinically, DDHAM was used to successfully treat anterior basement membrane dystrophy. Compared with DHAM and CHAM, DDHAM had significant positive effects on the cellular activities of HCECs in vitro, which may suggest greater ocular cell compatibility in vivo.
Keywords: anterior basement membrane dystrophy; biomaterial; decellularized scaffold; human amniotic membrane; human ocular epithelial cells; ocular surface.
© 2022 Celularity and The Authors. Journal of Biomedical Materials Research Part B: Applied Biomaterials published by Wiley Periodicals LLC.
Conflict of interest statement
Adam Kuehn, Desiree Long, Raja Sivalenka, Radoslaw A. Junka, Anna Gosiewska, Stephen A. Brigido, and Robert J. Hariri are salaried employees at Celularity Inc. Anish Shah functions as a key opinion leader for Celularity Inc. Yong Mao reports a grant from Celularity Inc. during the study. Nicole M. Protzman serves as an independent contractor for Celularity Inc. and reports personal fees from Celularity Inc. during the study. Nikita John has nothing to disclose.
Figures
References
-
- Leal‐Marin S, Kern T, Hofmann N, et al. Human amniotic membrane: a review on tissue engineering, application, and storage. J Biomed Mater Res B Appl Biomater. 2021;109:1198‐1215. - PubMed
-
- Liu J, Li L, Li X. Effectiveness of cryopreserved amniotic membrane transplantation in corneal ulceration: a meta‐analysis. Cornea. 2019;38:454‐462. - PubMed
-
- Fernandes M, Sridhar MS, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005;24:643‐653. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

