A post-marketing safety assessment of COVID-19 mRNA vaccination for serious adverse outcomes using administrative claims data linked with vaccination registry in a city of Japan
- PMID: 36371366
- PMCID: PMC9637511
- DOI: 10.1016/j.vaccine.2022.10.088
A post-marketing safety assessment of COVID-19 mRNA vaccination for serious adverse outcomes using administrative claims data linked with vaccination registry in a city of Japan
Abstract
Introduction: The safety profiles of COVID-19 vaccines are incompletely evaluated in Japan.
Objectives: To examine the risk of serious adverse effects after COVID-19 mRNA vaccination (BNT162b2 and mRNA-1273) in cohort studies and self-controlled case series (SCCS).
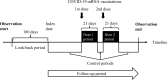
Methods: Using an administrative claims database linked with the COVID-19 vaccination registry in a city in Japan between September 2020 and September 2021, we identified health insurance enrolees aged ≥ 18 years. We evaluated the risk of acute myocardial infarction, appendicitis, Bell's palsy, convulsions/seizures, disseminated intravascular coagulation, immune thrombocytopenia, pulmonary embolism, haemorrhagic or ischemic stroke, venous thromboembolism, and all-cause mortality, 21 days following any COVID-19 mRNA vaccination, compared with non-vaccination periods. For the cohort studies, we estimated incidence rate ratios (IRRs) by Poisson regression and rate differences (IRDs) by weighted least-squares regression, adjusting for sex, age, and Charlson comorbidity index. We applied a modified SCCS design to appropriately treat outcome-dependent exposures. For the modified SCCS, we estimated within-subject IRRs by weighted conditional Poisson regression. Subgroup analyses stratified by sex and age were also conducted.
Results: We identified 184,491 enrolees [male: 87,218; mean (standard deviation) age: 64.2 (19.5) years] with 136,667 first and 127,322 s dose vaccinations. The risks of any outcomes did not increase in any analyses, except for the fact that the modified SCCS indicated an increased risk of pulmonary embolism after the first dose in women (within-subject IRR [95%CI]: 3.97 [1.18-13.32]).
Conclusion: The findings suggested that the COVID-19 mRNA vaccine was generally safe, whilst a signal of pulmonary embolism following the first dose of the COVID-19 mRNA vaccine was observed.
Keywords: Cohort study; Medical information database; Observational study; Pharmacoepidemiology; Self-controlled case series.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: YT has received consultant fees from Pharmaceuticals and Medical Devices Agency and EPARK, Inc. YT has received lecture fees from SAS Institute Japan Ltd. YT has been conducting a collaborative study, which is not related to this article, with Pfizer Inc. SO is a member of the Department of Eat-loss Medicine, a cooperative program between the University of Tokyo and ITO EN Ltd and received grants from KAKENHI, and Health, Labour, and Welfare Policy Research Grants, not related to the submitted work. No other potential competing interests relevant to this study are reported.
Figures

Similar articles
-
Messenger RNA Coronavirus Disease 2019 (COVID-19) Vaccination With BNT162b2 Increased Risk of Bell's Palsy: A Nested Case-Control and Self-Controlled Case Series Study.Clin Infect Dis. 2023 Feb 8;76(3):e291-e298. doi: 10.1093/cid/ciac460. Clin Infect Dis. 2023. PMID: 35675702 Free PMC article.
-
Evaluation of potential adverse events following COVID-19 mRNA vaccination among adults aged 65 years and older: Two self-controlled studies in the U.S.Vaccine. 2023 Jul 19;41(32):4666-4678. doi: 10.1016/j.vaccine.2023.06.014. Epub 2023 Jun 14. Vaccine. 2023. PMID: 37344261 Free PMC article.
-
Risk of serious adverse events after the BNT162b2, CoronaVac, and ChAdOx1 vaccines in Malaysia: A self-controlled case series study.Vaccine. 2022 Jul 30;40(32):4394-4402. doi: 10.1016/j.vaccine.2022.05.075. Epub 2022 Jun 3. Vaccine. 2022. PMID: 35667917 Free PMC article.
-
Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review.BMJ. 2022 Jul 13;378:e069445. doi: 10.1136/bmj-2021-069445. BMJ. 2022. PMID: 35830976 Free PMC article. Review.
-
Stroke Following Coronavirus Disease 2019 Vaccination: Evidence Based on Different Designs of Real-World Studies.J Infect Dis. 2023 Nov 11;228(10):1336-1346. doi: 10.1093/infdis/jiad306. J Infect Dis. 2023. PMID: 37536364
Cited by
-
Comparison of Transient and Persistent Adverse Events After COVID-19 Vaccination: A Retrospective Analysis.Cureus. 2024 Jun 28;16(6):e63410. doi: 10.7759/cureus.63410. eCollection 2024 Jun. Cureus. 2024. PMID: 39070394 Free PMC article.
-
Acute Appendicitis After COVID-19 Vaccines in Italy: A Self-Controlled Case Series Study.Drug Saf. 2024 Nov;47(11):1157-1169. doi: 10.1007/s40264-024-01462-0. Epub 2024 Jul 27. Drug Saf. 2024. PMID: 39068268 Free PMC article.
-
Determining the feasibility of linked claims and vaccination data for a COVID-19 vaccine pharmacoepidemiological study in Germany-RiCO feasibility study protocol.BMJ Open. 2024 Dec 22;14(12):e086074. doi: 10.1136/bmjopen-2024-086074. BMJ Open. 2024. PMID: 39806631 Free PMC article.
-
Individual trigger factors for hemorrhagic stroke: Evidence from case-crossover and self-controlled case series studies.Eur Stroke J. 2023 Sep;8(3):808-818. doi: 10.1177/23969873231173285. Epub 2023 May 8. Eur Stroke J. 2023. PMID: 37641550 Free PMC article. Review.
-
Analysis of the Association Between BNT162b2 mRNA COVID-19 Vaccination and Deaths Within 10 Days After Vaccination Using the Sex Ratio in Japan.Cureus. 2023 Dec 7;15(12):e50144. doi: 10.7759/cureus.50144. eCollection 2023 Dec. Cureus. 2023. PMID: 38077667 Free PMC article.
References
-
- Our World in Data. Coronavirus Pandemic (COVID-19). 2022. [Available from: https://ourworldindata.org/coronavirus#explore-the-global-situation.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

