A multivesicular body-like organelle mediates stimulus-regulated trafficking of olfactory ciliary transduction proteins
- PMID: 36371422
- PMCID: PMC9653401
- DOI: 10.1038/s41467-022-34604-y
A multivesicular body-like organelle mediates stimulus-regulated trafficking of olfactory ciliary transduction proteins
Abstract
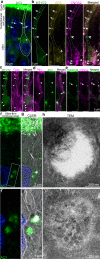
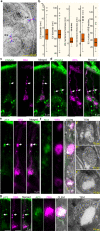
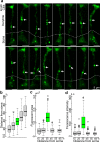
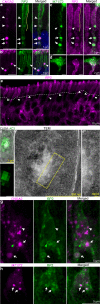
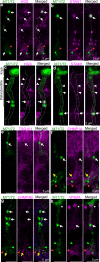
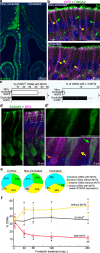
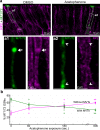
Stimulus transduction in cilia of olfactory sensory neurons is mediated by odorant receptors, Gαolf, adenylate cyclase-3, cyclic nucleotide-gated and chloride ion channels. Mechanisms regulating trafficking and localization of these proteins in the dendrite are unknown. By lectin/immunofluorescence staining and in vivo correlative light-electron microscopy (CLEM), we identify a retinitis pigmentosa-2 (RP2), ESCRT-0 and synaptophysin-containing multivesicular organelle that is not part of generic recycling/degradative/exosome pathways. The organelle's intraluminal vesicles contain the olfactory transduction proteins except for Golf subunits Gγ13 and Gβ1. Instead, Gβ1 colocalizes with RP2 on the organelle's outer membrane. The organelle accumulates in response to stimulus deprivation, while odor stimuli or adenylate cyclase activation cause outer membrane disintegration, release of intraluminal vesicles, and RP2/Gβ1 translocation to the base of olfactory cilia. Together, these findings reveal the existence of a dendritic organelle that mediates both stimulus-regulated storage of olfactory ciliary transduction proteins and membrane-delimited sorting important for G protein heterotrimerization.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Roizin L, Nishikawa K, Koizumi J, Keoseian S. The fine structure of the multivesicular body and their relationship to the ultracellular constituents of the central nervous system. J. Neuropathol. Exp. Neurol. 1967;26:223–249. - PubMed
-
- Hanson PI, Cashikar A. Multivesicular body morphogenesis. Annu Rev. Cell Dev. Biol. 2012;28:337–362. - PubMed
-
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

