SARS-CoV-2 mRNA vaccination of aplastic anemia patients is safe and effective
- PMID: 36371670
- PMCID: PMC9877677
- DOI: 10.1002/ajh.26780
SARS-CoV-2 mRNA vaccination of aplastic anemia patients is safe and effective
Conflict of interest statement
Authors declare that they have no competing interests.
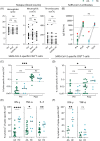
Figures
References
-
- Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172(2):187‐207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
