Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: A propensity score-adjusted analysis of a prospective cohort study
- PMID: 36371994
- PMCID: PMC9661716
- DOI: 10.1016/j.breast.2022.10.017
Adjuvant trastuzumab without chemotherapy for treating early HER2-positive breast cancer in older patients: A propensity score-adjusted analysis of a prospective cohort study
Abstract
Purpose: To gauge the effects of treatment practices on prognosis for older patients with HER2-positive early breast cancer, particularly to determine whether adjuvant trastuzumab alone can offer benefit over no adjuvant therapy. This is a prospective cohort study which accompanies the RESPECT that is a randomized-controlled trial (RCT).
Methods: Patients who declined the RCT were treated based on the physician's discretion. We studied the 1) trastuzumab-plus-chemotherapy group, 2) trastuzumab-monotherapy group, and 3) non-trastuzumab group (no therapy or anticancer therapy without trastuzumab). The primary endpoint was disease-free survival (DFS), which was compared using the propensity-score method. Relapse-free survival (RFS) and health-related quality of life (HRQoL) were assessed.
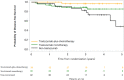
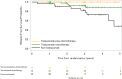
Results: We enrolled 123 patients aged over 70 years (median: 74.5). Treatment categories were: trastuzumab-plus-chemotherapy group (n = 36, 30%), trastuzumab-monotherapy group (n = 52, 43%), and non-trastuzumab group (n = 32, 27%). The 3-year DFS was 96.7% in trastuzumab-plus-chemotherapy group, 89.2% in trastuzumab-monotherapy group, and 82.5% in non-trastuzumab group. DFS in non-trastuzumab group was lower than in trastuzumab-plus-chemotherapy and trastuzumab-monotherapy groups (propensity-adjusted hazard ratio; HR: 3.29; 95% CI: 1.15-9.39; P = 0.026). The RFS in non-trastuzumab group was lower than in trastuzumab-plus-chemotherapy and trastuzumab-monotherapy groups (propensity-adjusted HR = 7.80; 95% CI: 2.32-26.2, P < 0.0001). There were no significant intergroup differences in the proportions of patients showing HRQoL deterioration at 36 months (P = 0.717).
Conclusion: Trastuzumab-treated patients had better prognoses than patients not treated with trastuzumab without deterioration of HRQoL. Trastuzumab monotherapy could be considered for older patients who reject chemotherapy.
Keywords: Breast cancer; HER2; Older; Trastuzumab; Without chemotherapy.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest YU reports honoraria for consulting from Chugai pharmaceutical Co., Ltd. TT reports honoraria for lectures from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Eisai Co., Ltd., Pfizer Japan Inc., Novartis Pharma K·K., AstraZeneca K·K., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K·K., and Daiichi Sankyo Co., Ltd. TN reports fees for Non-CME services and honoraria for lectures from Chugai pharmaceutical Co., Ltd., AstraZeneca K·K., Novartis Pharma K·K., Eli Lilly Japan K·K., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, and Eisai Co., Ltd. TM reports fees for non-CME services and honoraria for lectures from AstraZeneca K·K, Chugai pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K·K., Daiichi Sankyo Co., Ltd., Nippon Kayaku Co., Ltd., and Pfizer Japan Inc. HI reports honoraria for lectures from Chugai pharmaceutical Co., Ltd. HM reports honoraria from AstraZeneca K·K, Pfizer Japan Inc, Takeda Pharmaceutical Company Limited, Daiichi Sankyo Co., Ltd and Taiho Pharmaceutical Co., Ltd; and research grants from the Japanese government, Daiichi Sankyo Co., Ltd, Eisai Co., Ltd, Nippon Kayaku Co., Ltd and Pfizer Japan Inc, outside the submitted work.
Figures



References
-
- Sawaki M., Taira N., Uemura Y., Saito T., Baba S., Kobayashi K., et al. Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J Clin Oncol. 2020;38:3743–3752. - PubMed
-
- Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. - PubMed
-
- Cella D.F., Tulsky D.S., Gray G., Sarafian B., Linn E., Bonomi A., et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. - PubMed
-
- Eton D.T., Cella D., Yost K.J., Yount S.E., Peterman A.H., Neuberg D.S., et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. - PubMed
-
- Piccart-Gebhart M.J., Procter M., Leyland-Jones B., Goldhirsch A., Untch M., Smith I., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

