Genetic Mimicry Analysis Reveals the Specific Lipases Targeted by the ANGPTL3-ANGPTL8 Complex and ANGPTL4
- PMID: 36372100
- PMCID: PMC9852701
- DOI: 10.1016/j.jlr.2022.100313
Genetic Mimicry Analysis Reveals the Specific Lipases Targeted by the ANGPTL3-ANGPTL8 Complex and ANGPTL4
Abstract
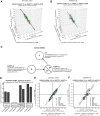
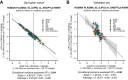
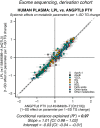
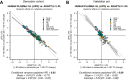
Angiopoietin-like proteins, ANGPTL3, ANGPTL4, and ANGPTL8, are involved in regulating plasma lipids. In vitro and animal-based studies point to LPL and endothelial lipase (EL, LIPG) as key targets of ANGPTLs. To examine the ANGPTL mechanisms for plasma lipid modulation in humans, we pursued a genetic mimicry analysis of enhancing or suppressing variants in the LPL, LIPG, lipase C hepatic type (LIPC), ANGPTL3, ANGPTL4, and ANGPTL8 genes using data on 248 metabolic parameters derived from over 110,000 nonfasted individuals in the UK Biobank and validated in over 13,000 overnight fasted individuals from 11 other European populations. ANGPTL4 suppression was highly concordant with LPL enhancement but not HL or EL, suggesting ANGPTL4 impacts plasma metabolic parameters exclusively via LPL. The LPL-independent effects of ANGPTL3 suppression on plasma metabolic parameters showed a striking inverse resemblance with EL suppression, suggesting ANGPTL3 not only targets LPL but also targets EL. Investigation of the impact of the ANGPTL3-ANGPTL8 complex on plasma metabolite traits via the ANGPTL8 R59W substitution as an instrumental variable showed a much higher concordance between R59W and EL activity than between R59W and LPL activity, suggesting the R59W substitution more strongly affects EL inhibition than LPL inhibition. Meanwhile, when using a rare and deleterious protein-truncating ANGPTL8 variant as an instrumental variable, the ANGPTL3-ANGPTL8 complex was very LPL specific. In conclusion, our analysis provides strong human genetic evidence that the ANGPTL3-ANGPTL8 complex regulates plasma metabolic parameters, which is achieved by impacting LPL and EL. By contrast, ANGPTL4 influences plasma metabolic parameters exclusively via LPL.
Keywords: angiopoietin-like proteins; cardiovascular disease; dyslipidemias; lipase/endothelial; lipase/hepatic; lipidomics; lipids; lipolysis and fatty acid metabolism; lipoprotein/metabolism; triglycerides.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Ginsberg H.N., Packard C.J., Chapman M.J., Boren J., Aguilar-Salinas C.A., Averna M., et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021;42:4791–4806. - PMC - PubMed
-
- Kersten S. Physiological regulation of lipoprotein lipase. Biochim. Biophys. Acta. 2014;1841:919–933. - PubMed
-
- Kersten S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 2019;30:205–211. - PubMed
-
- Kersten S., Mandard S., Tan N.S., Escher P., Metzger D., Chambon P., et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 2000;275:28488–28493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

