A tamoxifen-inducible Cre knock-in mouse for lens-specific gene manipulation
- PMID: 36372215
- PMCID: PMC9839650
- DOI: 10.1016/j.exer.2022.109306
A tamoxifen-inducible Cre knock-in mouse for lens-specific gene manipulation
Abstract
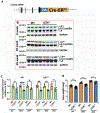
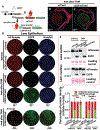
Mouse models are valuable tools in studying lens biology and biochemistry, and the Cre-loxP system is the most used technology for gene targeting in the lens. However, numerous genes are indispensable in lens development. The conventional knockout method either prevents lens formation or causes simultaneous cataract formation, hindering the studies of their roles in lens structure, growth, metabolism, and cataractogenesis during lens aging. An inducible Cre-loxP mouse line is an excellent way to achieve such a purpose. We established a lens-specific Cre ERT2 knock-in mouse (LCEK), an inducible mouse model for lens-specific gene targeting in a spatiotemporal manner. LCEK mice were created by in-frame infusion of a P2A-CreERT2 at the C-terminus of the last coding exon of the gene alpha A crystallin (Cryaa). LCEK mice express tamoxifen-inducible Cre recombinase uniquely in the lens. Through ROSAmT/mG and two endogenous genes (Gclc and Rbpj) targeting, we found no Cre recombinase leakage in the lens epithelium, but 50-80% leakage was observed in the lens cortex and nucleus. Administration of tamoxifen almost completely abolished target gene expression in both lens epithelium and cortex but only mildly enhanced gene deletion in the lens nucleus. Notably, no overt leakage of Cre activity was detected in developing LCEK lens when bred with mice carrying loxP floxed genes that are essential for lens development. This newly generated LCEK line will be a powerful tool to target genes in the lens for gene functions study in lens aging, posterior capsule opacification (PCO), and other areas requiring precision gene targeting.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest ZW, CH, JC, LG, and XF declare they have no conflict of interest.
Figures




Similar articles
-
High-fidelity Glucagon-CreER mouse line generated by CRISPR-Cas9 assisted gene targeting.Mol Metab. 2017 Jan 12;6(3):236-244. doi: 10.1016/j.molmet.2017.01.003. eCollection 2017 Mar. Mol Metab. 2017. PMID: 28271030 Free PMC article.
-
A mouse model with tamoxifen-inducible thyrocyte-specific cre recombinase activity.Genesis. 2014 Apr;52(4):333-40. doi: 10.1002/dvg.22740. Epub 2014 Jan 15. Genesis. 2014. PMID: 24395757
-
Expression of tamoxifen-inducible CRE recombinase in Lcn5-CreERT2 transgenic mouse caput epididymis.Mol Reprod Dev. 2017 Mar;84(3):257-264. doi: 10.1002/mrd.22772. Epub 2017 Mar 16. Mol Reprod Dev. 2017. PMID: 28029195
-
Conditional gene expression in the mouse inner ear using Cre-loxP.J Assoc Res Otolaryngol. 2012 Jun;13(3):295-322. doi: 10.1007/s10162-012-0324-5. Epub 2012 Apr 24. J Assoc Res Otolaryngol. 2012. PMID: 22526732 Free PMC article. Review.
-
Endothelial-Specific Cre Mouse Models.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2550-2561. doi: 10.1161/ATVBAHA.118.309669. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354251 Free PMC article. Review.
References
-
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S, 2005. Age-related cataract. Lancet 365, 599–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

