Evidence on the effectiveness of policies promoting price transparency - A systematic review
- PMID: 36372608
- PMCID: PMC10357344
- DOI: 10.1016/j.healthpol.2022.11.002
Evidence on the effectiveness of policies promoting price transparency - A systematic review
Abstract
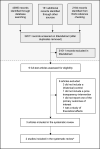
Policies promoting price transparency may be an important approach to control medicine prices and achieve better access to medicines. As part of a wider review, we aimed to systematically determine whether policies promoting price transparency are effective in managing the prices of pharmaceutical products. We searched for studies published between January 1, 2004 and October 10, 2019, comparing policies promoting price transparency against other interventions or a counterfactual. Eligible study designs included randomized trials, and non-randomized or quasi-experimental studies such as interrupted time-series (ITS), repeated measures (RM), and controlled before-after studies. Studies were eligible if they included at least one of the following outcomes: price (or expenditure as a proxy for price and volume), volume, availability or affordability of pharmaceutical products. The quality of the evidence was assessed using the GRADE methodology. A total of 32011 records were retrieved, two of which were eligible for inclusion. Although based on evidence from a single study, public disclosure of medicine prices may be effective in reducing prices of medicines short-term, with benefits possibly sustained long-term. Evidence on the impact of a cost-feedback approach to prescribers was inconclusive. No evidence was found for impact on the outcomes volume, availability or affordability. The overall lack of evidence on policies promoting price transparency is a clear call for further research.
Keywords: Medicine prices; Price transparency; Pricing policy; Systematic review.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest This systematic review was commissioned and funded by the World Health Organization.
Figures

Similar articles
-
Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD005979. doi: 10.1002/14651858.CD005979. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2014 Oct 16;(10):CD005979. doi: 10.1002/14651858.CD005979.pub2. PMID: 16625648 Updated.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Pharmaceutical policies: effects of regulating drug insurance schemes.Cochrane Database Syst Rev. 2022 May 3;5(5):CD011703. doi: 10.1002/14651858.CD011703.pub2. Cochrane Database Syst Rev. 2022. PMID: 35502614 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Interventions for preventing and reducing the use of physical restraints of older people in general hospital settings.Cochrane Database Syst Rev. 2022 Aug 25;8(8):CD012476. doi: 10.1002/14651858.CD012476.pub2. Cochrane Database Syst Rev. 2022. PMID: 36004796 Free PMC article.
Cited by
-
Patient Perspectives on the Current Paradigm of Non-Invasive Cardiac Testing Through an Emergency Department Observation Unit: A Survey Study.Cureus. 2025 Jun 3;17(6):e85296. doi: 10.7759/cureus.85296. eCollection 2025 Jun. Cureus. 2025. PMID: 40612835 Free PMC article.
-
Pricing and reimbursement mechanisms for advanced therapy medicinal products in 20 countries.Front Pharmacol. 2023 Nov 28;14:1199500. doi: 10.3389/fphar.2023.1199500. eCollection 2023. Front Pharmacol. 2023. PMID: 38089054 Free PMC article.
-
Effects of transparent online open procurement on prices, volumes, and costs of medicines: an interrupted time series study in Ningxia, China.Front Pharmacol. 2024 Aug 20;15:1362374. doi: 10.3389/fphar.2024.1362374. eCollection 2024. Front Pharmacol. 2024. PMID: 39228526 Free PMC article.
-
Role of biosimilar introduction on insulin glargine prices: a retrospective analysis in 28 European countries.BMJ Open. 2025 Jan 30;15(1):e090484. doi: 10.1136/bmjopen-2024-090484. BMJ Open. 2025. PMID: 39890142 Free PMC article.
-
India's Decision to Deny an Extension of Patent for Bedaquiline: A Public Health Imperative.Cureus. 2023 Nov 28;15(11):e49542. doi: 10.7759/cureus.49542. eCollection 2023 Nov. Cureus. 2023. PMID: 38156185 Free PMC article. Review.
References
-
- World Health Organization; Geneva: 2019. Fair Pricing Forum 2019 Meeting Report.https://www.who.int/medicines/access/fair_pricing/fair_price_report_2019... WHO/MVP/EMP/IAU/2019.09. Available from. [accessed 29/03/2021]
-
- World Health Organization . World Health Organization; Geneva: 2019. Seventy-Second World Health Assembly: Improving the transparency of markets for medicines, vaccines, and other health products.https://apps.who.int/gb/ebwha/pdf_files/WHA72/A72_R8-en.pdf Available from. [accessed 29/03/2021]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

