Cerebral Cortical Encephalitis in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
- PMID: 36372941
- PMCID: PMC10107670
- DOI: 10.1002/ana.26549
Cerebral Cortical Encephalitis in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
Abstract
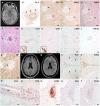
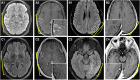
Cerebral cortical encephalitis (CCE) is a recently described myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) phenotype. In this observational retrospective study, we characterized 19 CCE patients (6.7% of our MOGAD cohort). Headache (n = 15, 79%), seizures (n = 13, 68%), and encephalopathy (n = 12, 63%) were frequent. Magnetic resonance imaging revealed unilateral (n = 12, 63%) or bilateral (n = 7, 37%) cortical T2 hyperintensity and leptomeningeal enhancement (n = 17, 89%). N-Methyl-D-aspartate receptor autoantibodies coexisted in 2 of 15 tested (13%). CCE pathology (n = 2) showed extensive subpial cortical demyelination (n = 2), microglial reactivity (n = 2), and inflammatory infiltrates (perivascular, n = 1; meningeal, n = 1). Most received high-dose steroids (n = 17, 89%), and all improved, but 3 had CCE relapses. This study highlights the CCE spectrum and provides insight into its pathogenesis. ANN NEUROL 2023;93:297-302.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
C.V.‐S., Y.G., E.S., M.M., K.N.K., P.M.E., V.R., J.‐M.T., A.B., C.F.L., report no conflicts of interest. J.J.C. has served as a consultant for Roche and UCB, which have upcoming treatment trials in MOGAD. S.L.‐C. and A.K. have served on an advisory board for Genentech/Roche, which has an upcoming treatment trial in MOGAD. D.D. has consulted for UCB, which has an upcoming treatment trial in MOGAD. S.J.P. reports grants, personal fees, and nonfinancial support from Roche/Genentech, and personal fees for consulting services from UCB, which have upcoming treatment trials in MOGAD. E.P.F. has participated in advisory boards for Roche and UCB who have upcoming treatment trials in MOGAD. E.P.F. has received funding from the NIH (R01NS113828). Mayo Clinic Laboratories offer commercial testing for MOG‐IgG, but none of the authors receive financial compensation for this.
Figures
References
-
- Budhram A, Mirian A, Le C, et al. Unilateral cortical FLAIR‐hyperintense lesions in anti‐MOG‐associated encephalitis with seizures (FLAMES): characterization of a distinct clinico‐radiographic syndrome. J Neurol 2019;266:2481–2487. - PubMed
-
- Yao T, Zeng Q, Xie Y, et al. Clinical analysis of adult MOG antibody‐associated cortical encephalitis. Mult Scler Relat Disord 2022;5:103727. - PubMed
-
- Fujimori J, Ogawa R, Murata T, et al. Decreased subcortical T2 FLAIR signal with cortical T2 FLAIR hyperintense lesions in unilateral cerebral cortical encephalitis with myelin oligodendrocyte glycoprotein antibody. Neuroimmunol Rep 2022;2:100096.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

