In vivo T1 mapping of neonatal brain tissue at 64 mT
- PMID: 36372971
- PMCID: PMC10099617
- DOI: 10.1002/mrm.29509
In vivo T1 mapping of neonatal brain tissue at 64 mT
Abstract
Purpose: Ultralow-field (ULF) point-of-care MRI systems allow image acquisition without interrupting medical provision, with neonatal clinical care being an important potential application. The ability to measure neonatal brain tissue T1 is a key enabling technology for subsequent structural image contrast optimization, as well as being a potential biomarker for brain development. Here we describe an optimized strategy for neonatal T1 mapping at ULF.
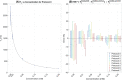
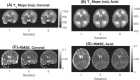
Methods: Examinations were performed on a 64-mT portable MRI system. A phantom validation experiment was performed, and a total of 33 in vivo exams were acquired from 28 neonates with postmenstrual age ranging from 31+4 to 49+0 weeks. Multiple inversion-recovery turbo spin-echo sequences were acquired with differing inversion and repetition times. An analysis pipeline incorporating inter-sequence motion correction generated proton density and T1 maps. Regions of interest were placed in the cerebral deep gray matter, frontal white matter, and cerebellum. Weighted linear regression was used to predict T1 as a function of postmenstrual age.
Results: Reduction of T1 with postmenstrual age is observed in all measured brain tissue; the change in T1 per week and 95% confidence intervals is given by dT1 = -21 ms/week [-25, -16] (cerebellum), dT1 = -14 ms/week [-18, -10] (deep gray matter), and dT1 = -35 ms/week [-45, -25] (white matter).
Conclusion: Neonatal T1 values at ULF are shorter than those previously described at standard clinical field strengths, but longer than those of adults at ULF. T1 reduces with postmenstrual age and is therefore a candidate biomarker for perinatal brain development.
Keywords: gray matter; neonatal; relaxometry; ultralow-field MRI; white matter.
© 2022 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Francesco Padormo was employed by the Guy′s & St. Thomas′ NHS Foundation Trust during experimental design, recruitment, all infant data collection, and before submission of the manuscript, but is now an employee of Hyperfine Inc. Lori Arlinghaus, John Pitts, Tianrui Luo, and Dingtian Zhang are employed by Hyperfine Inc.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

