Enhanced virulence and waning vaccine-elicited antibodies account for breakthrough infections caused by SARS-CoV-2 delta and beyond
- PMID: 36373096
- PMCID: PMC9635945
- DOI: 10.1016/j.isci.2022.105507
Enhanced virulence and waning vaccine-elicited antibodies account for breakthrough infections caused by SARS-CoV-2 delta and beyond
Abstract
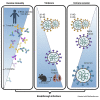
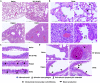
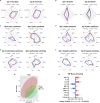
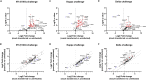
Here we interrogate the factors responsible for SARS-CoV-2 breakthrough infections in a K18-hACE2 transgenic mouse model. We show that Delta and the closely related Kappa variant cause viral pneumonia and severe lung lesions in K18-hACE2 mice. Human COVID-19 mRNA post-vaccination sera after the 2nd dose are significantly less efficient in neutralizing Delta/Kappa than early 614G virus in vitro and in vivo. By 5 months post-vaccination, ≥50% of donors lack detectable neutralizing antibodies against Delta and Kappa and all mice receiving 5-month post-vaccination sera die after the lethal challenges. Although a 3rd vaccine dose can boost antibody neutralization against Delta in vitro and in vivo, the mean log neutralization titers against the latest Omicron subvariants are 1/3-1/2 of those against the original 614D virus. Our results suggest that enhanced virulence, greater immune evasion, and waning of vaccine-elicited protection account for SARS-CoV-2 variants caused breakthrough infections.
Keywords: Immune response; Immunology; Virology.
Conflict of interest statement
The authors have no competing interests to declare.
Figures





References
-
- Gupta R., Charron J., Stenger C.L., Painter J., Steward H., Cook T.W., Faber W., Frisch A., Lind E., Bauss J., et al. SARS-CoV-2 (COVID-19) structural and evolutionary dynamicome: insights into functional evolution and human genomics. J. Biol. Chem. 2020;295:11742–11753. doi: 10.1074/jbc.RA120.014873. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

