Merging microfluidics with luminescence immunoassays for urgent point-of-care diagnostics of COVID-19
- PMID: 36373139
- PMCID: PMC9637550
- DOI: 10.1016/j.trac.2022.116814
Merging microfluidics with luminescence immunoassays for urgent point-of-care diagnostics of COVID-19
Abstract
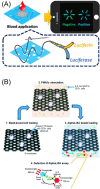
The Coronavirus disease 2019 (COVID-19) outbreak has urged the establishment of a global-wide rapid diagnostic system. Current widely-used tests for COVID-19 include nucleic acid assays, immunoassays, and radiological imaging. Immunoassays play an irreplaceable role in rapidly diagnosing COVID-19 and monitoring the patients for the assessment of their severity, risks of the immune storm, and prediction of treatment outcomes. Despite of the enormous needs for immunoassays, the widespread use of traditional immunoassay platforms is still limited by high cost and low automation, which are currently not suitable for point-of-care tests (POCTs). Microfluidic chips with the features of low consumption, high throughput, and integration, provide the potential to enable immunoassays for POCTs, especially in remote areas. Meanwhile, luminescence detection can be merged with immunoassays on microfluidic platforms for their good performance in quantification, sensitivity, and specificity. This review introduces both homogenous and heterogenous luminescence immunoassays with various microfluidic platforms. We also summarize the strengths and weaknesses of the categorized methods, highlighting their recent typical progress. Additionally, different microfluidic platforms are described for comparison. The latest advances in combining luminescence immunoassays with microfluidic platforms for POCTs of COVID-19 are further explained with antigens, antibodies, and related cytokines. Finally, challenges and future perspectives were discussed.
Keywords: COVID-19; Luminescence immunoassays; Microfluidic chips; POCTs.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










Similar articles
-
Materials for Microfluidic Immunoassays: A Review.Adv Healthc Mater. 2017 Aug;6(15). doi: 10.1002/adhm.201601403. Epub 2017 Mar 21. Adv Healthc Mater. 2017. PMID: 28322517 Review.
-
Microfluidics for COVID-19: From Current Work to Future Perspective.Biosensors (Basel). 2023 Jan 20;13(2):163. doi: 10.3390/bios13020163. Biosensors (Basel). 2023. PMID: 36831930 Free PMC article. Review.
-
Microfluidic-based blood immunoassays.J Pharm Biomed Anal. 2023 May 10;228:115313. doi: 10.1016/j.jpba.2023.115313. Epub 2023 Feb 24. J Pharm Biomed Anal. 2023. PMID: 36868029 Review.
-
Microfluidic chips for immunoassays.Annu Rev Anal Chem (Palo Alto Calif). 2013;6:119-41. doi: 10.1146/annurev-anchem-062012-092616. Epub 2013 Mar 14. Annu Rev Anal Chem (Palo Alto Calif). 2013. PMID: 23495732 Review.
-
Recent development of microfluidics-based platforms for respiratory virus detection.Biomicrofluidics. 2023 Apr 3;17(2):024104. doi: 10.1063/5.0135778. eCollection 2023 Mar. Biomicrofluidics. 2023. PMID: 37035101 Free PMC article.
Cited by
-
Digital and Analog Detection of SARS-CoV-2 Nucleocapsid Protein via an Upconversion-Linked Immunosorbent Assay.Anal Chem. 2023 Mar 14;95(10):4753-4759. doi: 10.1021/acs.analchem.2c05670. Epub 2023 Feb 27. Anal Chem. 2023. PMID: 36916131 Free PMC article.
-
Proteomics Methodologies: The Search of Protein Biomarkers Using Microfluidic Systems Coupled to Mass Spectrometry.Proteomes. 2023 May 10;11(2):19. doi: 10.3390/proteomes11020019. Proteomes. 2023. PMID: 37218924 Free PMC article. Review.
-
On-site bioaerosol sampling and detection in microfluidic platforms.Trends Analyt Chem. 2023 Jan;158:116880. doi: 10.1016/j.trac.2022.116880. Epub 2022 Dec 9. Trends Analyt Chem. 2023. PMID: 36514783 Free PMC article. Review.
References
-
- WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/(accessed 17 January, 2022).
-
- Zhou D., Dejnirattisai W., Supasa P., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Tuekprakhon A., Nutalai R., Wang B., Paesen G.C., Lopez-Camacho C., Slon-Campos J., Hallis B., Coombes N., Bewley K., Charlton S., Walter T.S., Skelly D., Lumley S.F., Dold C., Levin R., Dong T., Pollard A.J., Knight J.C., Crook D., Lambe T., Clutterbuck E., Bibi S., Flaxman A., Bittaye M., Belij-Rammerstorfer S., Gilbert S., James W., Carroll M.W., Klenerman P., Barnes E., Dunachie S.J., Fry E.E., Mongkolsapaya J., Ren J., Stuart D.I., Screaton G.R. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021;184(9):2348–2361. e2346. - PMC - PubMed
-
- Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., Huang B., Li D., Zhang R., Tian M., Fu L., Gao Y., Zhao X., Liu K., Qi J., Gao G.F., Wang P. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185(4):630–640. e610. - PMC - PubMed
-
- Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., Wang J., Wang Y., Niu X., Yang S., Liang H., Sun H., Li T., Yu Y., Cui Q., Liu S., Yang X., Du S., Zhang Z., Hao X., Shao F., Jin R., Wang X., Xiao J., Wang Y., Xie X.S. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
