Ozone application in different industries: A review of recent developments
- PMID: 36373160
- PMCID: PMC9637394
- DOI: 10.1016/j.cej.2022.140188
Ozone application in different industries: A review of recent developments
Abstract
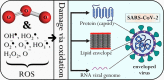
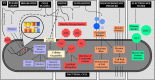
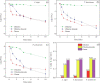
Ozone - a powerful antimicrobial agent, has been extensively applied for decontamination purposes in several industries (including food, water treatment, pharmaceuticals, textiles, healthcare, and the medical sectors). The advent of the COVID-19 pandemic has led to recent developments in the deployment of different ozone-based technologies for the decontamination of surfaces, materials and indoor environments. The pandemic has also highlighted the therapeutic potential of ozone for the treatment of COVID-19 patients, with astonishing results observed. The key objective of this review is to summarize recent advances in the utilisation of ozone for decontamination applications in the above-listed industries while emphasising the impact of key parameters affecting microbial reduction efficiency and ozone stability for prolonged action. We realise that aqueous ozonation has received higher research attention, compared to the gaseous application of ozone. This can be attributed to the fact that water treatment represents one of its earliest applications. Furthermore, the application of gaseous ozone for personal protective equipment (PPE) and medical device disinfection has not received a significant number of contributions compared to other applications. This presents a challenge for which the correct application of ozonation can mitigate. In this review, a critical discussion of these challenges is presented, as well as key knowledge gaps and open research problems/opportunities.
Keywords: Decontamination; Microbial inactivation; Ozone; Ozone dosage; Personal Protective Equipment (PPE).
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Guzel-Seydim Z.B., Greene A.K., Seydim A.C. Use of ozone in the food industry. LWT - Food Sci. Technol. 2004;37 doi: 10.1016/j.lwt.2003.10.014. - DOI
-
- Powell A., Scolding J.W.S. Direct application of ozone in aquaculture systems. Rev. Aquac. 2018;10 doi: 10.1111/raq.12169. - DOI
-
- Joseph C.G., Farm Y.Y., Taufiq-Yap Y.H., Pang C.K., Nga J.L.H., Li Puma G. Ozonation treatment processes for the remediation of detergent wastewater: A comprehensive review. J. Environ. Chem. Eng. 2021:9.
-
- Kim J.G., Yousef A.E., Dave S. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Prot. 1999:62. - PubMed
-
- Rice R.G., DeBrum M., Hook J., Cardis D., Tapp C. Microbiological benefits of ozone in laundering systems. Ozone Sci. Eng. 2009;31 doi: 10.1080/01919510903170419. - DOI
Publication types
LinkOut - more resources
Full Text Sources
