Newborn Screening for the Diagnosis and Treatment of Duchenne Muscular Dystrophy
- PMID: 36373292
- PMCID: PMC9881031
- DOI: 10.3233/JND-221535
Newborn Screening for the Diagnosis and Treatment of Duchenne Muscular Dystrophy
Abstract
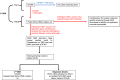
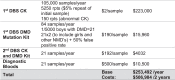
A pilot newborn screening (NBS) program for Duchenne muscular dystrophy (DMD) study proposes to assess the feasibility of the screening procedure, temporal course of the various steps of screening, and the public acceptability of the program. This is particularly vital to ascertain as DMD is considered a 'non-treatable' disease and thus does not fit the traditional criteria for newborn screening. However, modern perspectives of NBS for DMD are changing and point to possible net benefits for children and their families undertaking NBS for DMD. The aim of this workshop was to establish pathways for the successful implementation and evaluation of a pilot NBS for DMD program in Australia. Consensus was reached as to the rationale for, potential benefits, risks, barriers and facilitators of screening, alongside the establishment of screening protocols and clinical referral pathways.
Keywords: Duchenne muscular dystrophy; implementation; newborn screening; presymptomatic; translation.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Figures
References
-
- Towbin JA, Hejtmancik JF, Brink P, Gelb B, Zhu XM, Chamberlain JS, et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation 1993;87. - PubMed
-
- Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS From public health emergency to public health service: the implications of evolving criteria for newborn screening panels. Pediatrics. 2006;117(3):923–9. - PubMed
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–13. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

