Immunostimulants versus placebo for preventing exacerbations in adults with chronic bronchitis or chronic obstructive pulmonary disease
- PMID: 36373977
- PMCID: PMC9661939
- DOI: 10.1002/14651858.CD013343.pub2
Immunostimulants versus placebo for preventing exacerbations in adults with chronic bronchitis or chronic obstructive pulmonary disease
Abstract
Background: Individuals with chronic obstructive pulmonary disease (COPD) or chronic bronchitis may experience recurrent exacerbations, which negatively impact prognosis and quality of life, and can impose a significant socioeconomic burden on the individual and wider society. Immunostimulants are a broad category of therapies that may theoretically enhance non-specific immunity against several respiratory insults, thereby reducing exacerbation risk and severity. However, evidence to date for their use in this population is limited.
Objectives: To determine the efficacy of immunostimulants in preventing respiratory exacerbations in adults with chronic obstructive pulmonary disease, chronic bronchitis, or both.
Search methods: We used standard, extensive Cochrane search methods. The latest literature search was conducted on 25 January 2022. SELECTION CRITERIA: We included parallel randomised controlled trials (RCTs) that compared immunostimulant therapy, administered by any method and with the intention of preventing (rather than treating) exacerbations, with placebo for a minimum treatment duration of one month in adults with chronic bronchitis or COPD, or both. We excluded participants with other respiratory conditions. DATA COLLECTION AND ANALYSIS: We used standard Cochrane methods. Our primary outcomes were number of participants with no exacerbations during the study period and all-cause mortality, secondary outcomes were respiratory-related mortality, quality of life, number of participants requiring antibiotics, exacerbation duration, respiratory-related hospitalisation duration and adverse events/side effects. We used GRADE to assess certainty of evidence for each outcome.
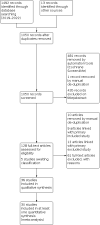
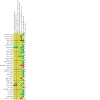
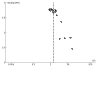
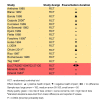
Main results: This review included 36 studies involving 6192 participants. Studies were published between 1981 and 2015. Duration ranged from three to 14 months. The mean age of study participants varied between 35.2 and 82 years. Twelve studies examined participants with COPD only. Seventeen studies reported baseline lung function values; most indicated a moderate-to-severe degree of airflow limitation. Nineteen studies indicated inclusion of participants with a mean baseline exacerbation frequency of two or more in the preceding year. Immunostimulants investigated were OM-85, AM3, RU41740 (Biostim), Ismigen, Diribiotine CK, thymomodulin, pidotimod, D53 (Ribomunyl), Lantigen B, Symbioflor, and hyaluronan; routes of administration were oral, sublingual, and subcutaneous. The risk of bias of the included studies was mostly low or unclear. Participants receiving immunostimulants for a mean duration of six months were slightly more likely to be free of exacerbations during that time (odds ratio (OR) 1.48, 95% confidence interval (CI) 1.15 to 1.90; 15 RCTs, 2961 participants; moderate-certainty evidence). The overall number needed to treat with immunostimulants for a mean of six months, to prevent one participant from experiencing an exacerbation, was 11 (95% CI 7 to 29). This outcome was associated with a moderate degree of unexplained heterogeneity (I2 = 53%). Type of immunostimulant, baseline lung function, baseline exacerbation frequency, treatment duration, and follow-up duration did not modify the effect size, although due to heterogeneity and limited study and participant numbers within some subgroups, the validity of the subgroup treatment effect estimates were uncertain. Immunostimulants probably result in little to no difference in all-cause mortality (OR 0.64, 95% CI 0.37 to 1.10; 5 RCTs, 1558 participants; moderate-certainty evidence) and respiratory-related mortality (OR 0.40, 95% CI 0.15 to 1.07; 2 RCTs, 735 participants; low-certainty evidence) compared to placebo; however, the effects were imprecise and data quality limited the certainty of these results. There was a small improvement in health-related quality of life, as measured by the St George's Respiratory Questionnaire (SGRQ), with immunostimulant compared to placebo (mean difference -4.59, 95% CI -7.59 to -1.59; 2 RCTs, 617 participants; very-low certainty evidence). The effect estimate just met the minimum clinically important difference (MCID) score of 4 units; however, the CI width means the possibility of a non-meaningful difference cannot be excluded. The pooled result from five studies indicated that immunostimulants likely reduce the number of participants requiring antibiotics over a mean duration of six months (OR 0.34, 95% CI 0.18 to 0.63; 542 participants; moderate-certainty evidence). This outcome had a low-to-moderate degree of heterogeneity (I2 = 38%), but the direction of effect was consistent across all studies. There was no evidence of a difference in the odds of experiencing an adverse event with immunostimulant compared to placebo, over a mean duration of six months (OR 1.01, 95% CI 0.84 to 1.21; 20 RCTs, 3780 participants; high-certainty evidence). The CI limits for the associated risk ratio (RR) did not cross thresholds for appreciable harm or benefit (RR 1.02, 95% CI 0.92 to 1.13). An additional seven studies reported no events rates in either study arm. Meta-analyses were not performed for the outcomes of exacerbation duration and respiratory-related hospitalisation duration, due to high levels of heterogeneity across the included studies (exacerbation duration: I2 = 92%; respiratory-related hospitalisation duration: I2 = 83%). Results from an effect direction plot and binomial probability test for exacerbation duration indicated that a significant proportion of studies (94% (95% CI 73% to 99%); P = 0.0002) favoured intervention, possibly indicating that immunostimulants are efficacious in reducing the mean exacerbation duration compared to placebo. However, the degree of uncertainty associated with this estimate remained high due to data quality and heterogeneity. Three studies reported mean duration of respiratory-related hospitalisation, two of which demonstrated a direction of effect that favoured immunostimulant over placebo.
Authors' conclusions: In participants with chronic bronchitis or COPD, we are moderately confident that treatment with immunostimulants is associated with a small reduction in the likelihood of having an exacerbation and a moderate reduction in the requirement for antibiotics. Low numbers of events limit interpretation of the effect of immunostimulants on all-cause and respiratory-related mortality. We are uncertain whether immunostimulants improve quality of life, and whether they are associated with a reduction in exacerbation and respiratory-related hospitalisation durations, although immunostimulants were generally associated with a positive effect direction in the studies that examined these outcomes. Immunostimulants appear to be safe and well-tolerated, and are not associated with an increased risk of adverse events.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
AF: none.
PP: none.
Figures





















Update of
- doi: 10.1002/14651858.CD013343
Similar articles
-
Prophylactic antibiotics for adults with chronic obstructive pulmonary disease: a network meta-analysis.Cochrane Database Syst Rev. 2021 Jan 15;1(1):CD013198. doi: 10.1002/14651858.CD013198.pub2. Cochrane Database Syst Rev. 2021. PMID: 33448349 Free PMC article.
-
Telehealth interventions: remote monitoring and consultations for people with chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Jul 20;7(7):CD013196. doi: 10.1002/14651858.CD013196.pub2. Cochrane Database Syst Rev. 2021. PMID: 34693988 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2021 Sep 8;9(9):CD013381. doi: 10.1002/14651858.CD013381.pub2. Cochrane Database Syst Rev. 2021. PMID: 34496032 Free PMC article.
-
Vitamin D for the management of chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2024 Sep 27;9(9):CD013284. doi: 10.1002/14651858.CD013284.pub2. Cochrane Database Syst Rev. 2024. PMID: 39329240
Cited by
-
MAIT cells are associated with responsiveness to neoadjuvant immunotherapy in COPD-associated NSCLC.Cancer Med. 2024 Mar;13(6):e7112. doi: 10.1002/cam4.7112. Cancer Med. 2024. PMID: 38509769 Free PMC article.
-
The protective role of muscone in the development of COPD.Front Immunol. 2025 Feb 17;16:1508879. doi: 10.3389/fimmu.2025.1508879. eCollection 2025. Front Immunol. 2025. PMID: 40034710 Free PMC article.
-
OM-85, a Bacterial Lysate, Reduces Pulmonary Nodule Malignant Probability: A Retrospective Study.Clin Respir J. 2025 Jul;19(7):e70109. doi: 10.1111/crj.70109. Clin Respir J. 2025. PMID: 40692186 Free PMC article.
-
Therapeutic and prophylactic effects of Qipian on COPD in mice: the role of lung and gut microbiota.Microbiol Spectr. 2025 Aug 5;13(8):e0196924. doi: 10.1128/spectrum.01969-24. Epub 2025 Jul 14. Microbiol Spectr. 2025. PMID: 40657918 Free PMC article.
References
References to studies included in this review
Alvarez‐Mon 2005 {published data only}
-
- Miravitlles M, Murio C, Morera J, Callol L, Alvarez-Sala JL, Alvarez-Mon M. Effect of AM3 on health-related quality of life in patients with chronic obstructive pulmonary disease belonging to risk groups. Medicina Clinica 2008;130(18):688-92. - PubMed
Alvarez‐Sala 2003 {published data only}
-
- Alvarez-Sala JL, Alvarez-Mon M. Treatment with Cellmune(TM) (AM3), an oral immunomodulator, increases the quality of life of chronic obstructive pulmonary disease (COPD) patients. Respiratory Care 2003;48(11):1071.
Anthoine 1985 {published data only}
-
- Anthoine D, Blaive B, Cabanieu G, Chretien J, Danrigal A, Ducreuzet C, et al. Double-blind study of Biostim in the prevention of superinfection in patients with chronic bronchopathy. Revue de Pneumologie Clinique 1985;41(3):213-7. - PubMed
Bisetti 1994 {published data only}
-
- Bisetti A, Ciappi G, Bariffi F, Catena E, Rocco V, Vaccaro L, et al. Evaluation of the efficacy of pidotimod in the exacerbations in patients affected with chronic bronchitis. Arzneimittel-Forschung 1994;44(12A):1499-502. - PubMed
Blaive 1982 {published data only}
-
- Blaive B, Demichelis B, Lemoigne F, Clary C, Macone F. Effectiveness of vaccination by ribosomal extracts in the preventive treatment of infections episodes in chronic bronchopathies. Medecine et Maladies Infectieuses 1982;12(8):475-9.
Bonde 1986 {published data only}
-
- Bonde J, Dahl R, Edelstein R, Kok-Jensen A, Lazer L, Punakivi L, et al. The effect of RU 41.740, an immune modulating compound, in the prevention of acute exacerbations in patients with chronic bronchitis. European Journal of Respiratory Diseases 1986;69(4):235-41. - PubMed
Bongiorno 1989 {published data only}
-
- Bongiorno A, Cataldo MG, Mazzola G, Indovina I. Controlled versus placebo study of the efficacy and tolerance of a new immunomodulator AM3 (immunoferon) in chronic bronchitis. Ciencia Medica 1989;6(9):357-62.
Braido 2015 {published data only}
-
- Braido F, Melioli G, Cazzola M, Fabbri L, Blasi F, Moretta L, et al. Sub-lingual administration of a polyvalent mechanical bacterial lysate (PMBL) in patients with moderate, severe, or very severe chronic obstructive pulmonary disease (COPD) according to the GOLD spirometric classification: a multicentre, double-blind, randomised, controlled, phase IV study (AIACE study: advanced Immunological Approach in COPD Exacerbation). Pulmonary Pharmacology & Therapeutics 2015;33:75-80. [DOI: 10.1016/j.pupt.2015.03.006] - DOI - PubMed
-
- EUCTR2007-000006-67-IT. Sub-lingual administration of a polyvalent mechanical bacterial lysate (PMBL) in patients with moderate or severe and very severe chronic obstructive pulmonary disease (COPD) according to GOLD classification: a multicenter, international, double blind, randomized, controlled, phase IV study. hwww.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-000006-67-IT (first received 8 June 2009). - PubMed
Carlo 1990 {published data only}
-
- Carlo M Sr, Carlo M Jr. Double blind study controlled with placebo of a new immunomodulator substance. Geriatrika 1990;6(6):65-9.
Catena 1992 {published data only}
-
- Catena E, Grassi C, Grossi E. Efficacy of treatment with thymomodulin in the profilaxis of exacerbations in COPD patients with cell-mediated deficit. Giornale Italiano della Malattie del Torace 1992;46(3):193-202.
Cazzola 2006 {published data only}
-
- Cazzola M. A new bacterial lysate protects by reducing infectious exacerbations in moderate to very severe COPD: a double-blind, randomized, placebo-controlled trial. Luglio 2006;6(3):191-9.
Ciaccia 1994 {published data only}
-
- Ciaccia A, Zavattini G, Ferri P. Pidotimod in the treatment of chronic bronchitis exacerbations. Giornale Italiano delle Malattie del Torace 1993;47(1-2):27-33.
-
- Ciaccia A. Pidotimod activity against chronic bronchitis exacerbations. Arzneimittel-Forschung 1994;44(12A):1516-20. - PubMed
Collet 1997 {published data only}
-
- Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. The PARI-IS Study Steering Committee and Research Group. Prevention of acute respiratory infection by an immunostimulant. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1719-24. [DOI: 10.1164/ajrccm.156.6.9612096] - DOI - PubMed
Cvoriscec 1989 {published data only}
Debbas 1990 {published data only}
-
- Debbas N, Derenne JP. Preventive effects of immunostimulating product on recurrent infections of chronic bronchitis in the elderly. Lung 1990;168:737-40. - PubMed
De Bernardi 1992 {published data only}
-
- De Bernardi M, Pedrinazzi PM, Re A, Colombo P, Fabiani A, Zanasi A. Immunostimulation induced by bacterial lysates in chronic bronchitis. A double blind placebo controlled clinical study. Acta Toxicologica et Therapeutica 1992;13(2):73-92.
Djuric 1989 {published data only}
-
- Djuric O, Mihailovic-Vucinic V, Stojcic V. Effect of Broncho-Vaxom on clinical and immunological parameters in patients with chronic obstructive bronchitis. A double-blind, placebo-controlled study. International Journal of Immunotherapy 1989;5(3):139-43.
EUCTR2007‐004702‐27‐DE {published data only}
-
- EUCTR2007-004702-27-DE. Multicentre, double-blind, placebo-controlled, randomised clinical study of Broncho-Vaxom® (Broncho-Munal®) for the protection from acute exacerbations of COPD. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-004702-27-DE (first received 7 December 2007).
Fietta 1988 {published data only}
-
- Fietta A, Bersani C, De Rose V, Mangiarotti P, Merlini C, Uccelli M, et al. Double-blind trial RU 41740 vs. placebo: immunological and clinical effects in a group of patients with chronic bronchitis. Respiration; International Review of Thoracic Diseases 1988;54(3):145-52. [DOI: 10.1159/000195515] - DOI - PubMed
Foschino 1995 {published data only}
-
- Foschino BM, Resta O, Cassano A, Altieri A, Guido P, Piti A, et al. Infectious exacerbations of chronic pulmonary diseases. Therapeutic effectiveness of immunomodulants. Minerva Pneumologica 1995;34(2):39-44.
Habermann 2001 {published data only}
-
- Habermann W, Zimmermann K, Skarabis H, Kunze R, Rusch V. The effect of a bacterial immunostimulant (human Enterococcus faecalis bacteria) on the occurrence of relapse in patients with chronic recurrent bronchitis. Arzneimittel-Forschung 2001;51(11):931-7. - PubMed
Hutas 1994 {published data only (unpublished sought but not used)}
-
- Hutas I, Kraszko P, Boszormenyi Nagy G. Immunomodulation therapy in chronic bronchitis (multicenter study). Orvosi Hetilap 1994;135(23):1251-4. - PubMed
Keller 1984 {published data only}
-
- Keller R, Hinz G. Effect of an oral polyvalent bacterial lysate (Broncho-Vaxom) in chronic bronchitis. Praxis und Klinik der Pneumologie EMT 1984;38(6):225-8. - PubMed
-
- Keller R. Multicenter double-blind study of the action of Broncho-Vaxom in chronic bronchitis. Schweizerische Medizinische Wochenschrift 1984;114(25):934-7. - PubMed
Li 2004 {published data only}
-
- Li J, Zheng JP, Yuan JP, Yang L, Luo DF, An JY, et al. Protection of oral immunostimulant bronchovaxom against with chronic obstructive pulmonary disease. Respirology 2005;10(3):A137.
-
- Li J, Zheng JP, Yuan JP, Zeng GQ, Zhong NS, Lin CY. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary disease. Chinese Medical Journal 2004;117(6):828-34. - PubMed
Menardo 1985 {published data only}
-
- Menardo JL, Dussol JP, Betbeder A. Immunotherapy of respiratory infection by oral immunomodulation in chronic obstructive bronchopneumopathy. Medecine et Hygiene 1985;43(1623):2722-6.
Messerli 1981 {published data only}
-
- Messerli C, Michetti F, Sauser-Hall P, Stäubli C, Taddei M, Weiss S, et al. Effect of a bacterial lysate (Broncho-Vaxom) in the therapy of chronic bronchitis: multi-center double-blind clinical trial. Revue Médicale de la Suisse Romande 1981;101(2):143-6. - PubMed
Olivieri 2011 {published data only}
-
- Olivieri D. Reducing exacerbations in COPD with OM-85: a multicentre, double-blind, placebo-controlled trial. European Respiratory Journal 2011;38(55):9s.
Orcel 1994 {published data only}
-
- Orcel B, Delclaux B, Baud M, Derenne JP. Oral immunization with bacterial extracts for protection against acute bronchitis in elderly institutionalized patients with chronic bronchitis. European Respiratory Journal 1994;7(3):446-52. - PubMed
-
- Orcel B, Delclaux B, Baud M, Derenne JP. Preventive effect of an immunomodulator, OM-85 BV, on acute exacerbations of chronic bronchitis in elderly patients. Preliminary results at six months in 291 patients. Revue des Maladies Respiratoires 1993;10(1):23-8. - PubMed
Orlandi 1983 {published data only}
-
- Orlandi O, Bruna S, Bagnasco G. Immunostimulation treatment with a bacteriolysate in recurring bacterial infections in chronic bronchitics. Minerva Pneumologica 1983;22:461-4.
Rico 1997 {published data only}
-
- Rico Mendez FG, Tellez Diaz E, Garcia Sancho C, Sanchez Diaz R, Yoma Medina J. Efficacy and prevention with thymomodulin on the numbers and duration of respiratory infectious episodes in adults with chronic obstructive lung disease. Revista Alergia Mexico 1997;44(4):93-101.
Rochemaure 1988 {published data only}
-
- Rochemaure J, Lehert P, Sauvaget J, Robillard M, Betbeder-Matibet A. Reduction using an immunomodulator of the level of respiratory infections in chronic bronchitis. Revue de Pneumologie Clinique 1988;44(1):43-7. - PubMed
-
- Rochemaure J, Lehert P, Sauvaget J, Robillard M, Murray N, Castanier JC. Usefulness of immunomodulating agents. Lancet 1987;2(8559):101. - PubMed
Soler 2007 {published data only}
-
- Soler M, Mutterlein R, Cozma G. Prevention of exacerbations in chronic bronchitis and COPD with OM-85, a double-blind, placebo-controlled trial. American Thoracic Society 100th International Conference; 2004 May 21-26; Orlando (FL). D22[Poster 506].
Tag 1993 {published data only}
-
- Tag E, Ashmawi S, Emam WE. Effect of a polyvalent bacterial extract, Broncho-Vaxom, in the prophylaxis of acute exacerbations of chronic bronchitis. European Journal of Clinical Research 1993;4:99-105.
Tang 2015 {published data only}
-
- Tang H, Fang Z, Xiu Q. Efficacy and safety of bacterial lysates in patients with chronic obstructive pulmonary disease and exacerbation. European Respiratory Journal 2011;38(55):599s.
Venge 1996 {published data only}
-
- Venge P, Pedersen B, Hakansson L, Hallgren R, Lindblad G, Dahl R. Subcutaneous administration of hyaluronan reduces the number of infectious exacerbations in patients with chronic bronchitis. American Journal of Respiratory and Critical Care Medicine 1996;153(1):312-6. [DOI: 10.1164/ajrccm.153.1.8542136] - DOI - PubMed
Xinogalos 1993 {published data only}
-
- Xinogalos S, Duratsos D, Varonos D. Clinical effectiveness of Broncho-Vaxom (BV) in patients with chronic obstructive pulmonary disease. International Journal of Immunotherapy 1993;9(2):135-42.
References to studies excluded from this review
Ahrens 1983 {published data only}
-
- Ahrens J, Wiedenbach M. Efficacy of the immunostimulant Broncho-Vaxom. Schweizerische Medizinische Wochenschrift 1984;114(25):932-4. - PubMed
-
- Ahrens J. Klinische wirksamkeit eines oralen immuntherapeutikums. Atemwegs-Lungenkr 1983;9:127-33.
Anonymous 1988 {published data only}
-
- Italian Group for the Study of Biological Immunomodulators. Effect of a purified glycoprotein substance extracted from Klebsiella pneumoniae in the clinical course of acute infections of the airways in patients with chronic bronchitis. Recenti Progressi in Medicina 1988;79(2):73-8. - PubMed
Anonymous 1993 {published data only}
-
- Anonymous. Pidotimod. Drugs of the future 1993;18(12):1163-4.
Anonymous 2008 {published data only}
-
- Anonymous. Proof of effectiveness of Pelargonium extract (Umckaloabo®) for the treatment of chronic bronchitis in a double-blind study. Schweizerische Zeitschrift fur Ganzheitsmedizin 2008;20(1):10-2.
Anthonisen 1997 {published data only}
-
- Anthonisen NR. OM-85 BV for COPD. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1713-4. - PubMed
Antonova 2004 {published data only}
-
- Antonova LP, Markova TP, Kurbatova EA. Naso-subcutaneous application of the polycomponent vaccine VP-4 for the treatment of patients with bronchial asthma and chronic obstructive bronchitis. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2004;6:36-40. - PubMed
Antonova 2008 {published data only}
-
- Antonova LP, Romanov VV, Averbakh MM. Experience with bronchomunal used in the combined treatment of patients with bronchial asthma and chronic obstructive pulmonary disease. Problemy Tuberkuleza I Boleznej Legkih 2008;4:8-11. - PubMed
Aparis 1987 {published data only}
-
- Aparis O, Berger M, Boisnic F, Cazard J, Delvaux H, Dewailly P, et al. Advantage of RU41740 associated with antibiotherapy in acute bronchial infections. A double blind versus placebo trial. Revue des Maladies Respiratoires 1987;4(6):66.
Banos 1997 {published data only}
Basacopol 1990 {published data only}
-
- Basacopol A, Carantino A, Calciu M. Broncho-Vaxom in the treatment and prevention of chronic pulmonary diseases. Clinical Trials Journal 1990;27(5):336-45.
-
- Basacopol A. Broncho-Vaxom in the treatment of chronic pulmonary lesions. Pneumoftiziologia 1992;41(1):25-30. - PubMed
Braido 2011 {published data only}
-
- Braido F, Traggiai E, Lanzilli G, Bazorro G, Folli C, Garelli V, et al. Modification of cell mediated immune-response in patients treated with a polyvalent mechanical bacterial lysate. European Respiratory Journal 2011;38:550.
Carre 1993 {published data only}
-
- Carre PC, Leophonte P. Role of anti-infective treatments in the management of chronic obstructive bronchopneumopathies. Revue des Maladies Respiratoires 1993;10(2):105-13. - PubMed
Carta 1994 {published data only}
-
- Carta P, Zedda S, Locci F, Lisci ML, Bande M, Del Giacco GS. Double-blind, randomized clinical trial of a bacterial immunomodulator in chronic obstructive bronchopneumonic infections. International Journal of Immunotherapy 1994;10(1):25-33.
Catena 1991 {published data only}
-
- Catena E. Thymopentin in the prevention of infective exacerbation in chronic bronchitis. Archivio Monaldi per le Malattie del Torace 1991;46(1-6):93-100. - PubMed
Cazzola 2009 {published data only}
-
- Cazzola M, Noschese P, Di Perna F. Value of adding a polyvalent bacterial lysate to therapy of COPD patients under regular treatment with salmeterol/fluticasone (SFC) [Abstract]. European Respiratory Society Annual Congress; 2009 Sep 12-16; Vienna, Austria. - PubMed
Centanni 1997 {published data only}
-
- Centanni S, Pregliasco F, Bonfatti C, Mensi C, Tarsia P, Guarnieri R, et al. Clinical efficacy of a vaccine-immunostimulant combination in the prevention of influenza in patients with chronic obstructive pulmonary disease and chronic asthma. Journal of Chemotherapy 1997;9(4):273-8. [DOI: 10.1179/joc.1997.9.4.273] - DOI - PubMed
Cogo 2003 {published data only}
-
- Cogo R, Ramponi A, Scivoletto G, Rippoli R. Prophylaxis for acute exacerbations of chronic bronchitis using an antibacterial sublingual vaccine obtained through mechanical lysis: a clinical and pharmacoeconomic study. Acta Bio Medica 2003;74(2):81-7. - PubMed
Cogo 2014 {published data only}
-
- Cogo R. Pidotimod activity in patients affected by COPD. Minerva Pneumologica 2014;53(1):21-6.
De Fenoyl 1986 {published data only}
-
- De Fenoyl O. An immunomodulating substance ('Biostim') obtained from Klebsiella pneumoniae. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1986;4(37):3-15.
Emanuele 1989 {published data only}
-
- Emanuele E, Corvaja E, Cumina T, Coppolino E. The immunomodulating effect of AM3 in the treatment of chronic bronchopathy. Rassegna di Medicina Interna 1989;10(1-2):25-31.
EUCTR2012‐003253‐28‐ES {published data only}
-
- EUCTR2012-003253-28-ES. Evaluation of the efficacy and safety of bactek in COPD. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-003253-28-ES (first received 29 October 2012).
EUCTR2013‐001940‐71‐GB {published data only}
-
- EUCTR2013-001940-71-GB. A study to investigate the effects of Broncho-Vaxom (OM-85 BV) on the immune system in patients with chronic obstructive pulmonary disease. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-001940-71-GB (first received 22 July 2013).
Fietta 1989 {published data only}
-
- Fietta A, Bersani C, Mangiarotti P, Merlini C, Uccelli M. Phagocytes and their pharmacological modulation in chronic bronchitis. International Journal of Immunopathology and Pharmacology 1989;2(2):115-22.
Fietta 1992 {published data only}
-
- Fietta AM, Merlini C, Uccelli M, Gialdroni Grassi G, Grassi C. Immunological and clinical effect of long-term oral treatment with RU 41740 in patients with chronic bronchitis: double-blind trial long-term versus standard dose regimen. Respiration; International Review of Thoracic Diseases 1992;59(5):253-8. [DOI: 10.1159/000196069] - DOI - PubMed
Fischer 1992 {published data only}
-
- Fischer H, Eckenberger HP, Aubel A, Kammereit A, Elsasser U, et al. Pravention von Infektrezidiven der oberen und unteren Luftwege. Atemw Lungenkrkh 1992;4:146-55. [DOI: ]
-
- Fischer H, Eckenberger HP, Aubel A, MacDonald TT, Challacombe SJ, Bland PW, et al. An oral bacterial lysate for the prevention of recurrent infections of the respiratory tract. Results of a double-blind placebo controlled 6 months study in 342 patients. In: MacDonald TT, Challacombe SJ, Bland PW, Stokes CR, Heatley RV, Mowat AM, editors(s). Advances in Mucosal Immunology. Norwell (MA): Kluwer Academic Publishers, 1990:351-2.
Gao 2014 {published data only}
Germouty 1986 {published data only}
-
- Germouty J. Immunotherapy of recurrent respiratory infections. Double-blind study of a new immunomodulator in 60 patients. Revue de Pneumologie Clinique 1986;42(4):207-13. - PubMed
Grassi 1988 {published data only}
-
- Grassi C, Bellero V, Donner CF, Fumagalli G, Orlandi O, Rimoldi A. Double-blind multicentre clinical study with Broncho-Vaxom in chronic bronchitis. European Respiratory Journal 1988;1(Suppl 2):252s.
Jia 2015 {published data only}
-
- Jia Z, Feng Z, Tian R, Wang Q, Wang L. Thymosin alpha1 plus routine treatment inhibit inflammatory reaction and improve the quality of life in AECOPD patients. Immunopharmacology and Immunotoxicology 2015;37(4):388-92. - PubMed
Kalinina 2003 {published data only}
-
- Kalinina EP, Zhuravskaia NS, Tsyvkina GI, Koziavina NV. Correction of immune disorders with neoselenium in patients with chronic bronchitis. Klinicheskaia Meditsina 2003;81(3):43-6. - PubMed
Khedr 1993 {published data only}
-
- Khedr MS. Clinical and immunological efficacy of Broncho-Vaxom(TM) in chronic bronchitis. A double-blind study. Acta Therapeutica 1993;19(1):49-60.
Koatz 2016 {published data only}
-
- Koatz AM, Zakin L, Ciceran A. Cost consequence of preventive treatment with OM 85 bacterial lysate compared to the same patients without OM 85 the previous year in allergic rhinitis, asthma and COPD in Argentina. Value in Health 2015;18(7):A498.
-
- Koatz AM. Evaluation of recurrent respiratory infections in patients with allergic rhinitis, asthma, and/or COPD pre-and post-treatment with bacterial lysate vaccines. Annals of Allergy, Asthma and Immunology 2014;113(5):A41.
Lacaille 1988 {published data only}
-
- Lacaille F. Administration of RU 41740, a prophylactic immunomodulator protecting against infection, during an episode of acute respiratory infection. Summarized results of 3 therapeutic trials. Presse Medicale 1988;17(28):1453-7. - PubMed
Mahashur 2002 {published data only}
-
- Mahashur AA, Pal M, Parikh HS, Karkera SG, Kukreja A. Study of the efficacy of immunomodulator imunocin, as an add-on therapy in the management of patients with chronic obstructive pulmonary disorder and bronchiectasis. Indian Practitioner 2002;55(6):393-9.
Malolepszy 1991 {published data only}
-
- Malolepszy J, Chyrek-Borowska S, Siwinska-Golebiowska HK, Rogala E, Roszkowski W. Multicentre clinical trial of Broncho-Vaxom in chronic bronchitis and asthma. Acta Therapeutica 1991;17(3):273-82.
Marcatili 1989 {published data only}
-
- Marcatili S, Catena E. Treatment of infective recurrences of chronic bronchopneumopathies with Klebsiella pneumoniae extracts. Arch Monaldi Mal Torace 1989;44(4-6):765-6. - PubMed
Matthys 2013 {published data only}
-
- Matthys H, Malek FA. Antibiotic use in patients with COPD receiving EPS 7630 as an add-on treatment. Atemwegs und Lungenkrankheiten 2015;41(1):27-34. [DOI: ]
-
- Matthys H, Pliskevich D, Reineke T, Malek FA. Health-related quality of life (HRQL) and patient-reported outcomes (PRO) in COPD patients receiving add-on-therapy with EPS 7630. European Respiratory Journal 2011;38(55):18s.
-
- Matthys H, Pliskevich DA, Malek FA, Tribanek M, Kieser M. Add-on-therapy in COPD with an herbal drug preparation. American Journal of Respiratory and Critical Care Medicine 2010;181:A4421.
Menon 2004 {published data only}
-
- Menon B. Effect of polyvalent bacterial extract (Broncho Vaxom) in the prophylaxis of acute exacerbation of COPD. Indian Journal of Allergy, Asthma and Immunology 2004;18(2):103.
Moniuszko 1991 {published data only}
-
- Moniuszko T, Rogalewska A, Chyrek-Borowska S. Clinical evaluation of Broncho-Vaxom efficiency. Polski Tygodnik Lekarski 1991;46(22-23):422-3. - PubMed
NCT01842360 {published data only}
-
- NCT01842360. Evaluation of the efficacy and safety of MV130 in chronic obstructive pulmonary disease (COPD). clinicaltrials.gov/show/NCT01842360 (first received 29 April 2013).
NCT02417649 {published data only}
-
- NCT02417649. Advanced immunological approach in COPD exacerbation. clinicaltrials.gov/show/NCT02417649 (first received 15 April 2015).
Nishantha 2014 {published data only (unpublished sought but not used)}
-
- Nishantha KM, Gunaratne HC, Yasaratne D, Medegedara RM. Polyvalent mechanical bacterial lysate in advanced COPD; a randomized controlled trial. Respirology 2014;19(Suppl 3):121. [DOI: 10.1111/resp.12417] - DOI
Nouvet 1989 {published data only}
-
- Nouvet G, Guerin JC, Leophonte P, Muir JF, Taytard A. Prevention of the recurrence of bronchial infection by ribomunyl tablet. Revue des Maladies Respiratoires 1989;6(Suppl 2):R105.
Obrecht 1975 {published data only}
-
- Obrecht U, Scherrer M. Diribiotine aerosols administered to patients with chronic bronchial obstruction. Double-blind study. Revue Medicale de la Suisse Romande 1975;95(5):345-59. - PubMed
Panchyshyna 1997 {published data only}
-
- Panchyshyna MV, Al-Qdemat YA, Panchyshyn JM, Petruh LI, Kazmirchuk TG. Effect of flurenizide on adaptive reactions in patients with chronic obstructive pulmonary disease. International Journal of Clinical Pharmacology Research 1997;17(1):47-52. - PubMed
Pozzi 1994 {published data only}
-
- Pozzi E, Dolcetti A, Orlandi O, Cirianni C, Moreo G, Piacenza G, et al. Pidotimod in the treatment of patients affected by bacterial exacerbations of chronic bronchitis. Arzneimittel-Forschung 1994;44(12A):1495-8. - PubMed
Rekalova 1992 {published data only}
-
- Rekalova EM. The immunomodulator kemantan in the treatment of patients with exacerbated chronic obstructive bronchitis. Likarska Sprava 1992;4:73-6. - PubMed
Ricci 2014 {published data only}
-
- Ricci R, Palmero C, Bazurro G, Riccio AM, Garelli V, Di Marco E, et al. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulmonary Pharmacology and Therapeutics 2014;27(1):109-13. [DOI: 10.1016/j.pupt.2013.05.006] - DOI - PubMed
Rico Mendez 1997 {published data only}
-
- Rico Mendez G, Massey L, Martinez Baez J, Mugica J, Martinez L. Prevention of respiratory tract infections (RTI) in patients with chronic obstructive pulmonary disease (COPD) with the use of oral bacterial lysates (OBL). European Respiratory Journal 1997;10(Suppl 25):202S.
Rimoldi 1990 {published data only}
-
- Rimoldi R, Clini V, Corsico R, Corda M, Bosisio E, Giura R, et al. Prophylaxis of acute infectious episodes in patients with chronic bronchitis with a newer immunomodulating agent: a prospective, controlled, randomized clinical trial. European Respiratory Journal 1990;3(Suppl 10):86S.
Romanski 1997 {published data only}
-
- Romanski B, Tomeczko J, Wilewska-Kubo T, Wierciski A, Tomaszewska B, Glejzer-Kichler O, et al. Therapeutic and immunomodulating properties of Peat Preparation To-Apa (PPT) administered to patients with recurrent respiratory tract infection in the course of nonatopic bronchial asthma and chronic obstructive bronchitis. International Review of Allergology and Clinical Immunology 1997;3(2):111-23.
Rutishauser 1998 {published data only}
-
- Rutishauser M, Pitzke P, Grevers G, Aubel A, Elsasser U, Kämmereit A. Use of a polyvalent bacterial lysate in patients with recurrent respiratory tract infections: results of a prospective, placebo-controlled, randomized, double-blind study. Advances in Therapy 1998;15:330-41. - PubMed
Shameen 2005 {published data only}
-
- Shameen M, Bhargav R, Ahmad Z, Sharma D, Shah NN. Effect of bronchovaxom on incidence and severity of upper respiratory tract infections in COPD patients. Indian Journal of Allergy Asthma and Immunology 2005;19(1):37-42.
Shmelev 1995 {published data only}
-
- Shmelev E, Khomenko A, Sokolova L, Philppov V, Kosmaidi G, Evguschenko G. Broncho-Vaxom in the management of chronic obstructive pulmonary diseases. European Respiratory Journal 1995;8(Suppl 19):236S.
Smirnova 2006 {published data only}
-
- Smirnova IaA, Trofimov VI, Shaporova NL. Efficacy of vaccine therapy with ribomunil in patients with chronic obstructive pulmonary disease. Terapevticheskii Arkhiv 2006;78(8):70-3. - PubMed
Sofia 1988 {published data only}
-
- Sofia M, Molino A, Mormile M, Di Benedetto G, De Simone F, Carratu L. Chronic bronchitis: a double-blind clinical trial for the evaluation of the therapeutical effectiveness of orally administered thymomodulin in the prevention of acute attacks. Giornale Italiano delle Malattie del Torace 1988;41(6):339-42.
-
- Sofia M. Chronic bronchitis: a double blind clinical trial for the evaluation of the therapeutical effectiveness of orally administered thymomodulin in the prevention of acute attacks. Indian Journal of Tuberculosis 1989;36(4):255.
Spiropoulos 1995 {published data only}
-
- Spiropoulos K, Lymberopoulos D, Ginopoulos P, Lavranos A. Effect of long-term therapy with oral Broncho-Vaxom in spirometry of chronic bronchitis patients. Lotta Contro la Tubercolosi e le Malattie Polmonari Sociali 1995;65(1-2):167-71.
Targowski 2005 {published data only}
-
- Targowski T, Jahnz-Rozyk K, Plusa T, Niedzialkowski P, Rozynska R. Influence of Broncho-Vaxom treatment on serum concentration of metalloproteinase-9 in patients with chronic obstructive pulmonary disease. Polski Merkuriusz Lekarski 2005;19(113):630-3. - PubMed
Viallat 1983 {published data only}
-
- Viallat JR, Costantini D, Boutin C, Farisse P. Double-blind trial of an immunomodulator of bacterial origin (Biostim) in the prevention of infectious episodes in chronic bronchitis. Poumon et le Coeur 1983;39(1):53-7. - PubMed
Zanussi 1990 {published data only}
-
- Zanussi C, Balsano F, Fagiolo U. Thymopentin in the prevention of recurrent respiratory infections in aged subjects with chronic bronchitis: a multicentre controlled randomized clinical trial. International Journal of Immunotherapy 1990;6(1):53-60.
Zheng 2008 {published data only}
-
- Zheng BX, Cheng DY, Xu G, Fan LL, Yang Y, Yang W. The prophylactic effect of thymosin alpha 1 on the acute exacerbation of chronic obstructive pulmonary disease. Sichuan da Xue Xue Bao. Yi Xue Ban 2008;39(4):588-90. - PubMed
References to studies awaiting assessment
Alvarez‐Sala 2004 {published data only}
-
- Alvarez-Sala JL, Alvarez-Mon M. Treatment with AM3 (Immunoferon), an oral immunomodulator, enhances the quality of life of patients with chronic obstructive pulmonary disease. Archivos de Bronconeumologia 2004;40(Suppl 2):37.
Berra 1988 {published data only}
-
- Berra A, Giella D, Galasso R, Matonti V, Lauriello G. Comparison of Klebsiella pneumoniae extract (Ru-41740) with placebo in acute infections in patients with chronic respiratory disease. Lotta Contro la Tuberculosi e le Malattie Polmonari Sociali 1988;58(3-4):727-30.
Magyar 1985 {published data only}
-
- Magyar P, Miskovits G, Nagy IA, Tarjan E, Zsiray M, Lantos A, et al. The therapeutic and preventive effects of BronchoVaxom, a lyophilized bacterial lysate, in chronic bronchitis: a double-blind placebo-controlled study. Pneumologia Hungarica 1985;38:293-303.
Shrewsbury 2014 {published data only (unpublished sought but not used)}
-
- Shrewsbury SB, Bjermer L, Vestbo J, Kuna P, Saarelainen P, Balint B, et al. The Flagship study: a 12-week phase II study to evaluate the efficacy and safety of AQX-1125 following exacerbations in patients with chronic obstructive pulmonary disease (COPD) by targeting the SHIP1 pathway [Abstract]. American Journal of Respiratory and Critical Care Medicine 2014;189:A2889.
Soler 2001 {published data only}
-
- Soler M, Mutterlein R. Multicentre, double-blind, placebo-controlled, randomised, clinical study of Broncho-Vaxom (OM-85) in patients suffering from acute exacerbations of chronic bronchitis or mild obstructive pulmonary disease. Clinical Study Reports 2001.
Additional references
Alyanakian 2006
-
- Alyanakian MA, Grela F, Aumeunier A, Chiavaroli C, Gouarin C, Bardel E, et al. Transforming growth factor-beta and natural killer T-cells are involved in the protective effect of a bacterial extract on type 1 diabetes. Diabetes 2006;55(1):179-85. [DOI: 10.2337/diabetes.55.01.06.db05-0189] - DOI - PubMed
ATS Foundation 2014
-
- American Thoracic Society Foundation. The global burden of lung disease. foundation.thoracic.org/news/global-burden.php (accessed 26 December 2018).
Baiardini 2005
-
- Baiardini I, Braido F, Fassio O, Tarantini F, Pasquali M, Tarchino F, et al. A new tool to assess and monitor the burden of chronic cough on quality of life: Chronic Cough Impact Questionnaire. Allergy 2005;60:482-8. - PubMed
Benetti 1994
-
- Benetti GP, Fugazza L, Stramba BM, Montalto F, Bombelli G, La Vecchia G, et al. Ex vivo evaluation of pidotimod activity on cell-mediated immunity. Drug Research 1994;44(12A):1476-9. [PMID: ] - PubMed
Bergemann 1994
-
- Bergemann R, Brandt A, Zoellner U, Donner CF. Preventive treatment of chronic bronchitis: a meta-analysis of clinical trials with a bacterial extract (OM-85 BV) and a cost-effectiveness analysis. Archivio Monaldi per le Malattie del Torace 1994;49(4):302-7. [PMID: ] - PubMed
Boissier 1988
-
- Boissier MC. Experimental immunopharmacology of RU 41740. La Presse Medicale 1988;17(28):1426-9. [PMID: ] - PubMed
Braido 2011
-
- Braido F, Schenone G, Pallestrini E, Reggiardo G, Cangemi G, Canonica GW, et al. The relationship between mucosal immunoresponse and clinical outcome in patients with recurrent upper respiratory tract infections treated with a mechanical bacterial lysate. Journal of Biological Regulators and Homeostatic Agents 2011;25(3):477-85. [PMID: ] - PubMed
Cazzola 2008
Cazzola 2012
Chong 2013
CMDD 2016
-
- Chinese Marketed Drug Database. Updated 28 March 2016. www.drugfuture.com/cndrug/ (accessed prior to 12 June 2022).
Collet 1997
-
- Collet JP, Shapiro P, Ernst P, Renzi T, Ducruet T, Robinson A. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. The PARI-IS Study Steering Committee and Research Group. Prevention of Acute Respiratory Infection by an Immunostimulant. American Journal of Respiratory and Critical Care Medicine 1997;156(6):1719-24. [DOI: 10.1164/ajrccm.156.6.9612096] - DOI - PubMed
Collet 2001
-
- Collet JP, Ducruet T, Haider S, Shapiro S, Robinson A, Renzi PM, et al. Economic impact of using an immunostimulating agent to prevent severe acute exacerbations in patients with chronic obstructive pulmonary disease. Canadian Respiratory Journal 2001;8(1):27-33. [DOI: 10.1155/2001/508015] - DOI - PubMed
Covidence [Computer program]
-
- Covidence. Melbourne, Australia: Veritas Health Innovation, (accessed prior to 1 September 2022). Available at covidence.org.
Cranston 2005
De Benedetto 2013
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. In: Available from training.cochrane.org/handbook.
Del‐Rio‐Navarro 2007
-
- Del-Rio-Navarro BE, González-Díaz S, Escalante-Domínguez AJ, Blandón-Vijil V. Immunostimulants in the prevention of respiratory infections. International Journal of Biotechnology 2007;9(3/4):246-60. [DOI: 10.1504/IJBT.2007.014245] - DOI
Del‐Rio‐Navarro 2012
Duchow 1992
EMA 2019
-
- European Medicines Agency. Assessment report: bacterial lysates-containing medicinal products indicated for respiratory conditions. Published 28 June 2019. www.ema.europa.eu/en/medicines/human/referrals/bacterial-lysates-contain... (accessed prior to 12 June 2022).
Ferris 1978
-
- Ferris BG. Epidemiology standardization project (American Thoracic Society). American Review of Respiratory Disease 1978;118:1-120. [PMID: ] - PubMed
GBD 2015
-
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. [DOI: 10.1016/S2213-2600(17)30293-X] - DOI - PMC - PubMed
Giovannini 2014
-
- Giovannini M, Salvini F, Riva E. Bacterial extracts as immunomodulators for the prevention of recurrent respiratory infections in children. Journal of Medical Microbiology and Diagnosis 2014;3:136. [DOI: 10.4172/2161-0703.1000136] - DOI
GOLD 2006
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. American Journal of Respiratory and Critical Care Medicine 2007;176:532-55. - PubMed
GOLD 2022
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2022 report. goldcopd.org/2022-gold-reports-2/ (accessed 6 March 2022).
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed prior to 17 January 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grossi 1989
-
- Grossi E, Blasi F, Nastri A, Montanari C. Predittivita di una scala di valutazione sintomatologica nella diagnosi delle nacutizzazioni in corso di BPCO: validazione mediante modello di analisi discriminante multivariata. Rassegna di Patologia Dell'Apparato Respiratorio 1989;IV(3):1-103.
Guyatt 1987
Hadden 1993
-
- Hadden JW. Immunostimulants. Trends in Pharmacological Sciences 1993;14(5):169-74. [PMID: ] - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Huber 2005
-
- Huber M, Mossmann M, Bessler WG. Th1-orientated immunological properties of the bacterial extract OM-85 BV. European Journal of Medical Research 2005;10(5):209-17. [PMID: ] - PubMed
Hurst 2010
Jinjuvadia 2017
-
- Jinjuvadia C, Jinjuvadia R, Mandapakala C, Durairajan N, Liangpunsakul S, Soubani AO. Trends in outcomes, financial burden, and mortality for acute exacerbation of chronic obstructive pulmonary disease (COPD) in the United States from 2002–2010. COPD: Journal of Chronic Obstructive Pulmonary Disease 2017;14(1):72-9. [DOI: 10.1080/15412555.2016.1199669] - DOI - PMC - PubMed
Jones 1992
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George's Respiratory Questionnaire. American Review of Respiratory Disease 1992;145(6):1321-7. - PubMed
Jones 2005
-
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75-9. - PubMed
Kanner 2001
-
- Kanner RE, Anthonisen NR, Connett JE, Lung Health Study Research Group. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. American Journal of Respiratory and Critical Care Medicine 2001;164(3):358-64. [DOI: 10.1164/ajrccm.164.3.2010017] - DOI - PubMed
Kew 2013
Kim 2011
Kim 2015
Köhnlein 2014
-
- Köhnlein T, Windisch W, Köhler D, Drabik A, Geiseler J, Hartl S, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respiratory Medicine 2014;2(9):698-705. [DOI: 10.1016/S2213-2600(14)70153-5] - DOI - PubMed
Lanzilli 2006
Lanzilli 2013
Li 2004
-
- Li J, Zheng JP, Yuan JP, Zeng GQ, Zhong NS, Lin CY. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary disease. Chinese Medical Journal 2004;117(6):828-34. [PMID: ] - PubMed
Lopez 2006
Marruchella 2018
Mauel 1989
Miller 2005
Mohan 2010
Moher 2009
Morandi 2011
-
- Morandi B, Agazzi A, D'Agostino A, Antonini F, Costa G, Sabatini F, et al. A mixture of bacterial mechanical lysates is more efficient than single strain lysate and of bacterial-derived soluble products for the induction of an activating phenotype in human dendritic cells. Immunology Letters 2011;138(1):86-91. [DOI: 10.1016/j.imlet.2011.03.006] - DOI - PubMed
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD006829. [DOI: 10.1002/14651858.CD006829.pub2] - DOI - PubMed
Navarro 2011
Ni 2015
Nikolova 2009
-
- Nikolova M, Stankulova D, Taskov H, Nenkov P, Maximov V, Petrunov B. Polybacterial immunomodulator respivax restores the inductive function of innate immunity in patients with recurrent respiratory infections. International Immunopharmacology 2009;9:425-32. [DOI: 10.1016/j.intimp.2009.01.004] - DOI - PubMed
Pan 2015
Papi 2006
-
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory and Critical Care Medicine 2006;173(10):1114-21. [DOI: 10.1164/rccm.200506-859OC] - DOI - PubMed
Pedraza‐Sánchez 2006
-
- Pedraza-Sánchez S, González-Hernández Y, Escobar-Gutiérrez A, Ramachandra L. The immunostimulant RU41740 from Klebsiella pneumoniae activates human cells in whole blood to potentially stimulate innate and adaptive immune responses. International Immunopharmacology 2006;6(4):635-46. [DOI: 10.1016/j.intimp.2005.10.006] - DOI - PubMed
Pivniouk 2022
-
- Pivniouk V, Pivniouk O, DeVries A, Uhrlaub JL, Michael A, Pivniouk D, et al. The OM-85 bacterial lysate inhibits SARS-CoV-2 infection of epithelial cells by downregulating SARS-CoV-2 receptor expression. Journal of Allergy and Clinical Immunology 2022;149(3):923-33.e6. [DOI: 10.1016/j.jaci.2021.11.019] - DOI - PMC - PubMed
Poole 2015
Review Manager 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhodes 1996
Rial 2004
-
- Rial A, Lens D, Betancor L, Benkiel H, Silva JS, Chabalgoity JA. Intranasal immunization with a colloid-formulated bacterial extract induces an acute inflammatory response in the lungs and elicits specific immune responses. Infection and Immunity 2004;72(5):2679-88. [DOI: 10.1128/IAI.72.5.2679-2688.2004] - DOI - PMC - PubMed
Ricci 2014
-
- Ricci R, Palmero C, Bazurro G, Riccio AM, Garelli V, Di Marco E, et al. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulmonary Pharmacology and Therapeutics 2014;27(1):109-13. [DOI: 10.1016/j.pupt.2013.05.006] - DOI - PubMed
Rohde 2003
Rossi 2003
-
- Rossi GA, Peri C, Raynal ME, Defilippi AC, Risso FM, Schenone G, et al. Naturally occurring immune response against bacteria commonly involved in upper respiratory tract infections: analysis of the antigen-specific salivary IgA levels. Immunology Letters 2003;86(1):85-91. [PMID: ] - PubMed
Rozy 2008
-
- Rozy A, Chorostowska-Wynimko J. Bacterial immunostimulants – mechanism of action and clinical application in respiratory diseases. Advances in Respiratory Medicine 2008;76(5):353-9. [PMID: ] - PubMed
Schünemann 2022
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Seemungal 1998
-
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(5):1418-22. [DOI: 10.1164/ajrccm.157.5.9709032] - DOI - PubMed
Singanayagam 2012
Soler‐Cataluña 2005
Spaner 2005
Sprenkle 2005
-
- Sprenkle MD, Niewoehner DE, MacDonald R, Rutks I, Wilt TJ. Clinical efficacy of OM-85 BV in COPD and chronic bronchitis: a systematic review. COPD: Journal of Chronic Obstructive Pulmonary Disease 2005;2(1):167-75. [PMID: ] - PubMed
Steurer‐Stey 2004
-
- Steurer-Stey C, Bachmann LM, Steurer J, Tramèr MR. Oral purified bacterial extracts in chronic bronchitis and COPD: systematic review. Chest 2004;126(5):1645-55. [10.1378/chest.126.5.1645] - PubMed
Tashkin 2008
Thomas 2020
-
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduced workload with minimal risk of missing studies: development and evaluation of a randomized controlled trial classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;133:140-51. [PMID: ] - PMC - PubMed
Tuthill 2013
-
- Tuthill CW, King RS. Thymosin alpha 1-A peptide immune modulator with a broad range of clinical applications. Journal of Clinical and Experimental Pharmacology 2013;3:133. [DOI: 10.4172/2161-1459.1000133] - DOI
Wedzicha 2007
WHO 2018
-
- World Health Organization. Projections of mortality and causes of death, 2016–2060. www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed prior to 17 January 2019).
WHO GHE 2016
-
- Global Health Estimates 2016: deaths by cause, age and sex, by country and by region, 2000–2016. www.who.int/healthinfo/global_burden_disease/estimates/en/ (accessed prior to 17 January 2019).
Woodhead 2011
Woodruff 2016
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

