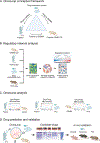
OncoLoop: A Network-Based Precision Cancer Medicine Framework
- PMID: 36374194
- PMCID: PMC9905319
- DOI: 10.1158/2159-8290.CD-22-0342
OncoLoop: A Network-Based Precision Cancer Medicine Framework
Abstract
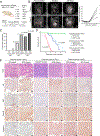
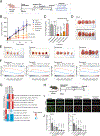
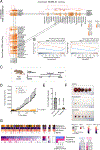
Prioritizing treatments for individual patients with cancer remains challenging, and performing coclinical studies using patient-derived models in real time is often unfeasible. To circumvent these challenges, we introduce OncoLoop, a precision medicine framework that predicts drug sensitivity in human tumors and their preexisting high-fidelity (cognate) model(s) by leveraging drug perturbation profiles. As a proof of concept, we applied OncoLoop to prostate cancer using genetically engineered mouse models (GEMM) that recapitulate a broad spectrum of disease states, including castration-resistant, metastatic, and neuroendocrine prostate cancer. Interrogation of human prostate cancer cohorts by Master Regulator (MR) conservation analysis revealed that most patients with advanced prostate cancer were represented by at least one cognate GEMM-derived tumor (GEMM-DT). Drugs predicted to invert MR activity in patients and their cognate GEMM-DTs were successfully validated in allograft, syngeneic, and patient-derived xenograft (PDX) models of tumors and metastasis. Furthermore, OncoLoop-predicted drugs enhanced the efficacy of clinically relevant drugs, namely, the PD-1 inhibitor nivolumab and the AR inhibitor enzalutamide.
Significance: OncoLoop is a transcriptomic-based experimental and computational framework that can support rapid-turnaround coclinical studies to identify and validate drugs for individual patients, which can then be readily adapted to clinical practice. This framework should be applicable in many cancer contexts for which appropriate models and drug perturbation data are available. This article is highlighted in the In This Issue feature, p. 247.
©2022 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest:
None of the other authors report any conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA238005/CA/NCI NIH HHS/United States
- U01 CA261822/CA/NCI NIH HHS/United States
- S10 OD012351/OD/NIH HHS/United States
- P01 CA265768/CA/NCI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- U01 CA217858/CA/NCI NIH HHS/United States
- S10 OD021764/OD/NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
- P01 CA163227/CA/NCI NIH HHS/United States
- R00 CA194287/CA/NCI NIH HHS/United States
- U01 CA272610/CA/NCI NIH HHS/United States
- U54 CA209997/CA/NCI NIH HHS/United States
- P50 CA097186/CA/NCI NIH HHS/United States
- R01 CA173481/CA/NCI NIH HHS/United States
- P01 CA221757/CA/NCI NIH HHS/United States
- R01 CA183929/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

