Suppression of CCL2 angiocrine function by adrenomedullin promotes tumor growth
- PMID: 36374225
- PMCID: PMC9665902
- DOI: 10.1084/jem.20211628
Suppression of CCL2 angiocrine function by adrenomedullin promotes tumor growth
Abstract
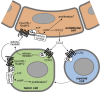
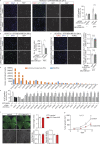
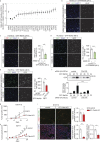
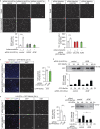
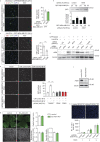
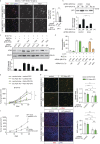
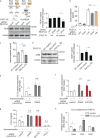
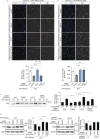
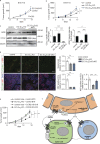
Within the tumor microenvironment, tumor cells and endothelial cells regulate each other. While tumor cells induce angiogenic responses in endothelial cells, endothelial cells release angiocrine factors, which act on tumor cells and other stromal cells. We report that tumor cell-derived adrenomedullin has a pro-angiogenic as well as a direct tumor-promoting effect, and that endothelium-derived CC chemokine ligand 2 (CCL2) suppresses adrenomedullin-induced tumor cell proliferation. Loss of the endothelial adrenomedullin receptor CALCRL or of the G-protein Gs reduced endothelial proliferation. Surprisingly, tumor cell proliferation was also reduced after endothelial deletion of CALCRL or Gs. We identified CCL2 as a critical angiocrine factor whose formation is inhibited by adrenomedullin. Furthermore, CCL2 inhibited adrenomedullin formation in tumor cells through its receptor CCR2. Consistently, loss of endothelial CCL2 or tumor cell CCR2 normalized the reduced tumor growth seen in mice lacking endothelial CALCRL or Gs. Our findings show tumor-promoting roles of adrenomedullin and identify CCL2 as an angiocrine factor controlling adrenomedullin formation by tumor cells.
© 2022 Nakayama et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures

Similar articles
-
CCL2/CCR2 Regulates the Tumor Microenvironment in HER-2/neu-Driven Mammary Carcinomas in Mice.PLoS One. 2016 Nov 7;11(11):e0165595. doi: 10.1371/journal.pone.0165595. eCollection 2016. PLoS One. 2016. PMID: 27820834 Free PMC article.
-
CCL2 in the Tumor Microenvironment.Adv Exp Med Biol. 2021;1302:1-14. doi: 10.1007/978-3-030-62658-7_1. Adv Exp Med Biol. 2021. PMID: 34286437
-
Hypoxia-induced CCL2/CCR2 axis in adipose-derived stem cells (ADSCs) promotes angiogenesis by human dermal microvascular endothelial cells (HDMECs) in flap tissues.J Physiol Biochem. 2023 May;79(2):327-339. doi: 10.1007/s13105-023-00944-6. Epub 2023 Feb 14. J Physiol Biochem. 2023. PMID: 36786974
-
Roles of CCL2-CCR2 Axis in the Tumor Microenvironment.Int J Mol Sci. 2021 Aug 8;22(16):8530. doi: 10.3390/ijms22168530. Int J Mol Sci. 2021. PMID: 34445235 Free PMC article. Review.
-
The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials.Int J Mol Sci. 2022 Mar 23;23(7):3485. doi: 10.3390/ijms23073485. Int J Mol Sci. 2022. PMID: 35408846 Free PMC article. Review.
Cited by
-
A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis.Vaccines (Basel). 2025 May 30;13(6):586. doi: 10.3390/vaccines13060586. Vaccines (Basel). 2025. PMID: 40573917 Free PMC article.
-
Hypoxia stimulates CTC-platelet cluster formation to promote breast cancer metastasis.iScience. 2024 Mar 20;27(5):109547. doi: 10.1016/j.isci.2024.109547. eCollection 2024 May 17. iScience. 2024. PMID: 38660400 Free PMC article.
-
Adrenomedullin in Tumorigenesis and Cancer Progression.Int J Mol Sci. 2025 Jun 10;26(12):5552. doi: 10.3390/ijms26125552. Int J Mol Sci. 2025. PMID: 40565016 Free PMC article. Review.
-
Blood flow-induced angiocrine signals promote organ growth and regeneration.Bioessays. 2025 Feb;47(2):e2400207. doi: 10.1002/bies.202400207. Epub 2024 Nov 11. Bioessays. 2025. PMID: 39529434 Free PMC article. Review.
References
-
- Abramoff, M.D., Magalhaes P.J., and Ram S.J.. 2004. Image processing with ImageJ. Biophot. Int. 11:36–42
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

