Predictors of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: A Prespecified Secondary Analysis of the STROKE AF Randomized Clinical Trial
- PMID: 36374508
- PMCID: PMC9664367
- DOI: 10.1001/jamaneurol.2022.4038
Predictors of Atrial Fibrillation in Patients With Stroke Attributed to Large- or Small-Vessel Disease: A Prespecified Secondary Analysis of the STROKE AF Randomized Clinical Trial
Abstract
Importance: The Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) trial found that approximately 1 in 8 patients with recent ischemic stroke attributed to large- or small-vessel disease had poststroke atrial fibrillation (AF) detected by an insertable cardiac monitor (ICM) at 12 months. Identifying predictors of AF could be useful when considering an ICM in routine poststroke clinical care.
Objective: To determine the association between commonly assessed risk factors and poststroke detection of new AF in the STROKE AF cohort monitored by ICM.
Design, setting, and participants: This was a prespecified analysis of a randomized (1:1) clinical trial that enrolled patients between April 1, 2016, and July 12, 2019, with primary follow-up through 2020 and mean (SD) duration of 11.0 (3.0) months. Eligible patients were selected from 33 clinical research sites in the US. Patients had an index stroke attributed to large- or small-vessel disease and were 60 years or older or aged 50 to 59 years with at least 1 additional stroke risk factor. A total of 496 patients were enrolled, and 492 were randomly assigned to study groups (3 did not meet inclusion criteria, and 1 withdrew consent). Patients in the ICM group had the index stroke within 10 days before insertion. Data were analyzed from October 8, 2021, to January 28, 2022.
Interventions: ICM monitoring vs site-specific usual care (short-duration external cardiac monitoring).
Main outcomes and measures: The ICM device automatically detects AF episodes 2 or more minutes in length; episodes were adjudicated by an expert committee. Cox regression multivariable modeling included all parameters identified in the univariate analysis having P values <.10. AF detection rates were calculated using Kaplan-Meier survival estimates.
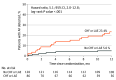
Results: The analysis included the 242 participants randomly assigned to the ICM group in the STROKE AF study. Among 242 patients monitored with ICM, 27 developed AF (mean [SD] age, 66.6 [9.3] years; 144 men [60.0%]; 96 [40.0%] women). Two patients had missing baseline data and exited the study early. Univariate predictors of AF detection included age (per 1-year increments: hazard ratio [HR], 1.05; 95% CI, 1.01-1.09; P = .02), CHA2DS2-VASc score (per point: HR, 1.54; 95% CI, 1.15-2.06; P = .004), chronic obstructive pulmonary disease (HR, 2.49; 95% CI, 0.86-7.20; P = .09), congestive heart failure (CHF; with preserved or reduced ejection fraction: HR, 6.64; 95% CI, 2.29-19.24; P < .001), left atrial enlargement (LAE; HR, 3.63; 95% CI, 1.55-8.47; P = .003), QRS duration (HR, 1.02; 95% CI, 1.00-1.04; P = .04), and kidney dysfunction (HR, 3.58; 95% CI, 1.35-9.46; P = .01). In multivariable modeling (n = 197), only CHF (HR, 5.06; 95% CI, 1.45-17.64; P = .05) and LAE (HR, 3.32; 1.34-8.19; P = .009) remained significant predictors of AF. At 12 months, patients with CHF and/or LAE (40 of 142 patients) had an AF detection rate of 23.4% vs 5.0% for patients with neither (HR, 5.1; 95% CI, 2.0-12.8; P < .001).
Conclusions and relevance: Among patients with ischemic stroke attributed to large- or small-vessel disease, CHF and LAE were associated with a significantly increased risk of poststroke AF detection. These patients may benefit most from the use of ICMs as part of a secondary stroke prevention strategy. However, the study was not powered for clinical predictors of AF, and therefore, other clinical characteristics may not have reached statistical significance.
Trial registration: ClinicalTrials.gov Identifier: NCT02700945.
Conflict of interest statement
Figures
References
-
- Bernstein RA, Kamel H, Granger CB, et al. ; STROKE-AF Investigators . Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE AF randomized clinical trial. JAMA. 2021;325(21):2169-2177. doi:10.1001/jama.2021.6470 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

