Human Metastatic Cholangiocarcinoma Patient-Derived Xenografts and Tumoroids for Preclinical Drug Evaluation
- PMID: 36374558
- PMCID: PMC9873249
- DOI: 10.1158/1078-0432.CCR-22-2551
Human Metastatic Cholangiocarcinoma Patient-Derived Xenografts and Tumoroids for Preclinical Drug Evaluation
Abstract
Purpose: Cholangiocarcinoma (CCA) is usually diagnosed at advanced stages, with limited therapeutic options. Preclinical models focused on unresectable metastatic CCA are necessary to develop rational treatments. Pathogenic mutations in IDH1/2, ARID1A/B, BAP1, and BRCA1/2 have been identified in 30%-50% of patients with CCA. Several types of tumor cells harboring these mutations exhibit homologous recombination deficiency (HRD) phenotype with enhanced sensitivity to PARP inhibitors (PARPi). However, PARPi treatment has not yet been tested for effectiveness in patient-derived models of advanced CCA.
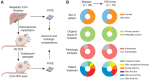
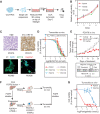
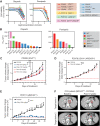
Experimental design: We have established a collection of patient-derived xenografts from patients with unresectable metastatic CCA (CCA_PDX). The CCA_PDXs were characterized at both histopathologic and genomic levels. We optimized a protocol to generate CCA tumoroids from CCA_PDXs. We tested the effects of PARPis in both CCA tumoroids and CCA_PDXs. Finally, we used the RAD51 assay to evaluate the HRD status of CCA tissues.
Results: This collection of CCA_PDXs recapitulates the histopathologic and molecular features of their original tumors. PARPi treatments inhibited the growth of CCA tumoroids and CCA_PDXs with pathogenic mutations of BRCA2, but not those with mutations of IDH1, ARID1A, or BAP1. In line with these findings, only CCA_PDX and CCA patient biopsy samples with mutations of BRCA2 showed RAD51 scores compatible with HRD.
Conclusions: Our results suggest that patients with advanced CCA with pathogenic mutations of BRCA2, but not those with mutations of IDH1, ARID1A, or BAP1, are likely to benefit from PARPi therapy. This collection of CCA_PDXs provides new opportunities for evaluating drug response and prioritizing clinical trials.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
- 1078-0432. doi: 10.1158/1078-0432.CCR-29-2-HI
References
-
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. . Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. - PubMed
-
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. . SEER Cancer Statistics Review, 1975–2018, NCI. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site; 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

