Long-term progression of retinal degeneration in a preclinical model of CLN7 Batten disease as a baseline for testing clinical therapeutics
- PMID: 36374771
- PMCID: PMC9626557
- DOI: 10.1016/j.ebiom.2022.104314
Long-term progression of retinal degeneration in a preclinical model of CLN7 Batten disease as a baseline for testing clinical therapeutics
Abstract
Background: Batten disease is characterized by cognitive and motor impairment, retinal degeneration, and seizures leading to premature death. Recent studies have shown efficacy for a gene therapy approach for CLN7 Batten disease. This gene therapy approach is promising to treat cognitive and motor impairment, but is not likely to delay vision loss. Additionally, the natural progression of retinal degeneration in CLN7 Batten disease patients is not well-known.
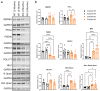
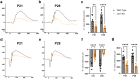
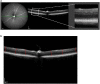
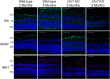
Methods: We performed visual examinations on five patients with CLN7 Batten disease and found that patients were far progressed in degeneration within their first five years of life. To better understand the disease progression, we characterized the retina of a preclinical mouse model of CLN7 Batten disease, through the age at which mice present with paralysis and premature death.
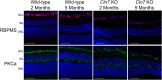
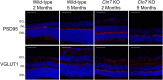
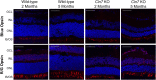
Findings: We found that this preclinical model shows signs of photoreceptor to bipolar synaptic defects early, and displays rod-cone dystrophy with late loss of bipolar cells. This vision loss could be followed not only via histology, but using clinical live imaging similar to that used in human patients.
Interpretation: Natural history studies of rare paediatric neurodegenerative conditions are complicated by the rapid degeneration and limited availability of patients. Characterization of degeneration in the preclinical model allows for future experiments to better understand the mechanisms underlying the retinal disease progression in order to find therapeutics to treat patients, as well as to evaluate these therapeutic options for future human clinical trials.
Funding: Van Sickle Family Foundation Inc., NIHP30EY030413, Morton Fichtenbaum Charitable Trust and 5T32GM131945-03.
Keywords: Bipolar cell degeneration; Electroretinography; Mfsd8; Neuronal ceroid lipofuscinoses; Optical coherence tomography; Photoreceptor synapse.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Ms. Rowe has nothing to disclose. Dr. Chen has nothing to disclose. Ms. Nettesheim has nothing to disclose. Mr..ßIssioui has nothing to disclose. Dr. Dong has nothing to disclose. Ms. Hu has nothing to disclose. Dr. Messahel has nothing to disclose. Dr. Kayani has nothing to disclose. Dr. Gray has nothing to disclose. Dr. Wert has nothing to disclose.
Figures









References
-
- Nita D.A., Mole S.E., Minassian B.A. Neuronal ceroid lipofuscinoses. Epileptic Disord. 2016;18(S2):73–88. - PubMed
-
- Chang C.H. Neuronal ceroid lipofuscinoses. 2017. https://emedicine.medscape.com/article/1178391-overview
-
- Wisniewski K.E., Kida E., Golabek A.A., Kaczmarski W., Connell F., Zhong N. Neuronal ceroid lipofuscinoses: classification and diagnosis. Adv Genet. 2001;45:1–34. - PubMed
-
- Wisniewski K.E., Zhong N., Philippart M. Pheno/genotypic correlations of neuronal ceroid lipofuscinoses. Neurology. 2001;57(4):576–581. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

