Enhanced recombinant protein production in CHO cell continuous cultures under growth-inhibiting conditions is associated with an arrested cell cycle in G1/G0 phase
- PMID: 36374852
- PMCID: PMC9662745
- DOI: 10.1371/journal.pone.0277620
Enhanced recombinant protein production in CHO cell continuous cultures under growth-inhibiting conditions is associated with an arrested cell cycle in G1/G0 phase
Abstract
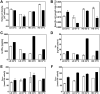
Low temperature and sodium butyrate (NaBu) are two of the most used productivity-enhancing strategies in CHO cell cultures during biopharmaceutical manufacturing. While these two approaches alter the balance in the reciprocal relationship between cell growth and productivity, we do not fully understand their mechanisms of action beyond a gross cell growth inhibition. Here, we used continuous culture to evaluate the differential effect of low temperature and NaBu supplementation on CHO cell performance and gene expression profile. We found that an increase in cell-productivity under growth-inhibiting conditions was associated with the arrest of cells in the G1/G0 phase. A transcriptome analysis revealed that the molecular mechanisms by which low temperature and NaBu arrested cell cycle in G1/G0 differed from each other through the deregulation of different cell cycle checkpoints and regulators. The individual transcriptome changes in pattern observed in response to low temperature and NaBu were retained when these two strategies were combined, leading to an additive effect in arresting the cell cycle in G1/G0 phase. The findings presented here offer novel molecular insights about the cell cycle regulation during the CHO cell bioprocessing and its implications for increased recombinant protein production. This data provides a background for engineering productivity-enhanced CHO cell lines for continuous manufacturing.
Copyright: © 2022 Avello et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

