Structure-Based Discovery of Small-Molecule Inhibitors of the Autocatalytic Proliferation of α-Synuclein Aggregates
- PMID: 36374974
- PMCID: PMC9811465
- DOI: 10.1021/acs.molpharmaceut.2c00548
Structure-Based Discovery of Small-Molecule Inhibitors of the Autocatalytic Proliferation of α-Synuclein Aggregates
Abstract
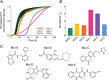
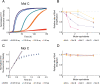
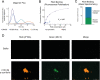
The presence of amyloid fibrils of α-synuclein is closely associated with Parkinson's disease and related synucleinopathies. It is still very challenging, however, to systematically discover small molecules that prevent the formation of these aberrant aggregates. Here, we describe a structure-based approach to identify small molecules that specifically inhibit the surface-catalyzed secondary nucleation step in the aggregation of α-synuclein by binding to the surface of the amyloid fibrils. The resulting small molecules are screened using a range of kinetic and thermodynamic assays for their ability to bind α-synuclein fibrils and prevent the further generation of α-synuclein oligomers. This study demonstrates that the combination of structure-based and kinetic-based drug discovery methods can lead to the identification of small molecules that selectively inhibit the autocatalytic proliferation of α-synuclein aggregates.
Keywords: Parkinson’s disease, α-synuclein; computational docking; kinetic-based small-molecule discovery; protein aggregation; structure-based small-molecule discovery.
Conflict of interest statement
The authors declare the following competing financial interest(s): Sara Linse, Tuomas P. J. Knowles, Johnny Habchi and Michele Vendruscolo are founders of Wren Therapeutics. Andrea Possenti, Benedetta Mannini, Roxine Staats are currently employees of Wren Therapeutics. Sean Chia and Rodrigo Cataldi have been employees of Wren Therapeutics. Robert I. Horne is a consultant for Wren Therapeutics Magdalena Nowinska has been a consultant for Wren Therapeutics.
Figures


References
-
- Nichols E.; Szoeke C. E. I.; Vollset S. E.; Abbasi N.; Abd-Allah F.; Abdela J.; Aichour M. T. E.; Akinyemi R. O.; Alahdab F.; Asgedom S. W.; Awasthi A.; Barker-Collo S. L.; Baune B. T.; Béjot Y.; Belachew A. B.; Bennett D. A.; Biadgo B.; Bijani A.; Bin Sayeed M. S.; Brayne C.; Carpenter D. O.; Carvalho F.; Catalá-López F.; Cerin E.; Choi J. Y. J.; Dang A. K.; Degefa M. G.; Djalalinia S.; Dubey M.; Duken E. E.; Edvardsson D.; Endres M.; Eskandarieh S.; Faro A.; Farzadfar F.; Fereshtehnejad S. M.; Fernandes E.; Filip I.; Fischer F.; Gebre A. K.; Geremew D.; Ghasemi-Kasman M.; Gnedovskaya E. V.; Gupta R.; Hachinski V.; Hagos T. B.; Hamidi S.; Hankey G. J.; Haro J. M.; Hay S. I.; Irvani S. S. N.; Jha R. P.; Jonas J. B.; Kalani R.; Karch A.; Kasaeian A.; Khader Y. S.; Khalil I. A.; Khan E. A.; Khanna T.; Khoja T. A. M.; Khubchandani J.; Kisa A.; Kissimova-Skarbek K.; Kivimäki M.; Koyanagi A.; Krohn K. J.; Logroscino G.; Lorkowski S.; Majdan M.; Malekzadeh R.; März W.; Massano J.; Mengistu G.; Meretoja A.; Mohammadi M.; Mohammadi-Khanaposhtani M.; Mokdad A. H.; Mondello S.; Moradi G.; Nagel G.; Naghavi M.; Naik G.; Nguyen L. H.; Nguyen T. H.; Nirayo Y. L.; Nixon M. R.; Ofori-Asenso R.; Ogbo F. A.; Olagunju A. T.; Owolabi M. O.; Panda-Jonas S.; Passos V. M. d. A.; Pereira D. M.; Pinilla-Monsalve G. D.; Piradov M. A.; Pond C. D.; Poustchi H.; Qorbani M.; Radfar A.; Reiner R. C.; Robinson S. R.; Roshandel G.; Rostami A.; Russ T. C.; Sachdev P. S.; Safari H.; Safiri S.; Sahathevan R.; Salimi Y.; Satpathy M.; Sawhney M.; Saylan M.; Sepanlou S. G.; Shafieesabet A.; Shaikh M. A.; Sahraian M. A.; Shigematsu M.; Shiri R.; Shiue I.; Silva J. P.; Smith M.; Sobhani S.; Stein D. J.; Tabarés-Seisdedos R.; Tovani-Palone M. R.; Tran B. X.; Tran T. T.; Tsegay A. T.; Ullah I.; Venketasubramanian N.; Vlassov V.; Wang Y. P.; Weiss J.; Westerman R.; Wijeratne T.; Wyper G. M. A.; Yano Y.; Yimer E. M.; Yonemoto N.; Yousefifard M.; Zaidi Z.; Zare Z.; Vos T.; Feigin V. L.; Murray C. J. L. Global, Regional, and National Burden of Alzheimer’s Disease and Other Dementias, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. 10.1016/S1474-4422(18)30403-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

