Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation
- PMID: 36375056
- PMCID: PMC9704719
- DOI: 10.1073/pnas.2208886119
Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation
Abstract
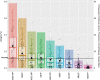
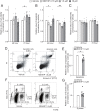
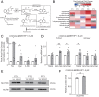
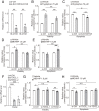
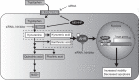
Uterine leiomyoma is the most common tumor in women and causes severe morbidity in 15 to 30% of reproductive-age women. Epidemiological studies consistently indicate a correlation between leiomyoma development and exposure to endocrine-disrupting chemical phthalates, especially di-(2-ethylhexyl) phthalate (DEHP); however, the underlying mechanisms are unknown. Here, among the most commonly encountered phthalate metabolites, we found the strongest association between the urine levels of mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), the principal DEHP metabolite, and the risk of uterine leiomyoma diagnosis (n = 712 patients). The treatment of primary leiomyoma and smooth muscle cells (n = 29) with various mixtures of phthalate metabolites, at concentrations equivalent to those detected in urine samples, significantly increased cell viability and decreased apoptosis. MEHHP had the strongest effects on both cell viability and apoptosis. MEHHP increased cellular tryptophan and kynurenine levels strikingly and induced the expression of the tryptophan transporters SLC7A5 and SLC7A8, as well as, tryptophan 2,3-dioxygenase (TDO2), the key enzyme catalyzing the conversion of tryptophan to kynurenine that is the endogenous ligand of aryl hydrocarbon receptor (AHR). MEHHP stimulated nuclear localization of AHR and up-regulated the expression of CYP1A1 and CYP1B1, two prototype targets of AHR. siRNA knockdown or pharmacological inhibition of SLC7A5/SLC7A8, TDO2, or AHR abolished MEHHP-mediated effects on leiomyoma cell survival. These findings indicate that MEHHP promotes leiomyoma cell survival by activating the tryptophan-kynurenine-AHR pathway. This study pinpoints MEHHP exposure as a high-risk factor for leiomyoma growth, uncovers a mechanism by which exposure to environmental phthalate impacts leiomyoma pathogenesis, and may lead to the development of novel druggable targets.
Keywords: aryl hydrocarbon receptor; endocrine-disrupting chemicals; leiomyoma; phthalate; tryptophan.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Bulun S. E., Uterine fibroids. N. Engl. J. Med. 369, 1344–1355 (2013). - PubMed
-
- Faerstein E., Szklo M., Rosenshein N., Risk factors for uterine leiomyoma: A practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am. J. Epidemiol. 153, 1–10 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

