PAHSAs reduce cellular senescence and protect pancreatic beta cells from metabolic stress through regulation of Mdm2/p53
- PMID: 36375063
- PMCID: PMC9704710
- DOI: 10.1073/pnas.2206923119
PAHSAs reduce cellular senescence and protect pancreatic beta cells from metabolic stress through regulation of Mdm2/p53
Abstract
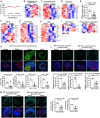
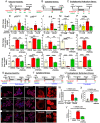
Senescence in pancreatic beta cells plays a major role in beta cell dysfunction, which leads to impaired glucose homeostasis and diabetes. Therefore, prevention of beta cell senescence could reduce the risk of diabetes. Treatment of nonobese diabetic (NOD) mice, a model of type 1 autoimmune diabetes (T1D), with palmitic acid hydroxy stearic acids (PAHSAs), a novel class of endogenous lipids with antidiabetic and antiinflammatory effects, delays the onset and reduces the incidence of T1D from 82% with vehicle treatment to 35% with PAHSAs. Here, we show that a major mechanism by which PAHSAs protect islets of the NOD mice is by directly preventing and reversing the initial steps of metabolic stress-induced senescence. In vitro PAHSAs increased Mdm2 expression, which decreases the stability of p53, a key inducer of senescence-related genes. In addition, PAHSAs enhanced expression of protective genes, such as those regulating DNA repair and glutathione metabolism and promoting autophagy. We demonstrate the translational relevance by showing that PAHSAs prevent and reverse early stages of senescence in metabolically stressed human islets by the same Mdm2 mechanism. Thus, a major mechanism for the dramatic effect of PAHSAs in reducing the incidence of type 1 diabetes in NOD mice is decreasing cellular senescence; PAHSAs may have a similar benefit in humans.
Keywords: cellular senescence; diabetes; lipids; metabolic stress; pancreatic islets.
Conflict of interest statement
Competing interest statement: I.S. and B.B.K. are inventors on patents related to the FAHFAs.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

