Efficacy and Safety of Trametinib Monotherapy or in Combination With Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma
- PMID: 36375115
- PMCID: PMC9870224
- DOI: 10.1200/JCO.22.01000
Efficacy and Safety of Trametinib Monotherapy or in Combination With Dabrafenib in Pediatric BRAF V600-Mutant Low-Grade Glioma
Abstract
Purpose: BRAF V600 mutations occur in many childhood cancers, including approximately 20% of low-grade gliomas (LGGs). Here, we describe a phase I/II study establishing pediatric dosing and pharmacokinetics of trametinib with or without dabrafenib, as well as efficacy and safety in a disease-specific cohort with BRAF V600-mutant LGG; other cohorts will be reported elsewhere.
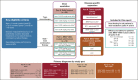
Methods: This is a four-part, phase I/II study (ClinicalTrials.gov identifier: NCT02124772) in patients age < 18 years with relapsed/refractory malignancies: trametinib monotherapy dose finding (part A) and disease-specific expansion (part B), and dabrafenib + trametinib dose finding (part C) and disease-specific expansion (part D). The primary objective assessed in all patients in parts A and C was to determine pediatric dosing on the basis of steady-state pharmacokinetics. Disease-specific efficacy and safety (across parts A-D) were secondary objectives.
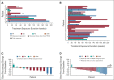
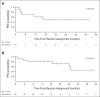
Results: Overall, 139 patients received trametinib (n = 91) or dabrafenib + trametinib (n = 48). Trametinib dose-limiting toxicities in > 1 patient (part A) included mucosal inflammation (n = 3) and hyponatremia (n = 2). There were no dose-limiting toxicities with combination therapy (part C). The recommended phase II dose of trametinib, with or without dabrafenib, was 0.032 mg/kg once daily for patients age < 6 years and 0.025 mg/kg once daily for patients age ≥ 6 years; dabrafenib dosing in the combination was as previously identified for monotherapy. In 49 patients with BRAF V600-mutant glioma (LGG, n = 47) across all four study parts, independently assessed objective response rates were 15% (95% CI, 1.9 to 45.4) for monotherapy (n = 13) and 25% (95% CI, 12.1 to 42.2) for combination (n = 36). Adverse event-related treatment discontinuations were more common with monotherapy (54% v 22%).
Conclusion: The trial design provided efficient evaluation of pediatric dosing, safety, and efficacy of single-agent and combination targeted therapy. Age-based and weight-based dosing of trametinib with or without dabrafenib achieved target concentrations with manageable safety and demonstrated clinical efficacy and tolerability in BRAF V600-mutant LGG.
Conflict of interest statement
No other potential conflicts of interest were reported.
Figures
Comment in
-
Advances in the treatment of BRAF-mutant low-grade glioma with MAPK inhibitors.Transl Pediatr. 2024 Mar 27;13(3):513-517. doi: 10.21037/tp-23-541. Epub 2024 Mar 18. Transl Pediatr. 2024. PMID: 38590382 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

