Treating Transthyretin Amyloidosis via Adeno-Associated Virus Vector Delivery of Meganucleases
- PMID: 36375122
- PMCID: PMC9700363
- DOI: 10.1089/hum.2022.061
Treating Transthyretin Amyloidosis via Adeno-Associated Virus Vector Delivery of Meganucleases
Abstract
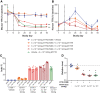
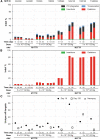
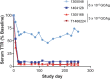
Transthyretin amyloidosis (ATTR) is a progressive and fatal disease caused by transthyretin (TTR) amyloid fibril accumulation in tissues, which disrupts organ function. As the TTR protein is primarily synthesized by the liver, liver transplantation can cure familial ATTR but is not an option for the predominant age-related wild-type ATTR. Approved treatment approaches include TTR stabilizers and an RNA-interference therapeutic, but these require regular re-administration. Gene editing could represent an effective one-time treatment. We evaluated adeno-associated virus (AAV) vector-delivered, gene-editing meganucleases to reduce TTR levels. We used engineered meganucleases targeting two different sites within the TTR gene. AAV vectors expressing TTR meganuclease transgenes were first tested in immunodeficient mice expressing the human TTR sequence delivered using an AAV vector and then against the endogenous TTR gene in rhesus macaques. Following a dose of 3 × 1013 genome copies per kilogram, we detected on-target editing efficiency of up to 45% insertions and deletions (indels) in the TTR genomic DNA locus and >80% indels in TTR RNA, with a concomitant decrease in serum TTR levels of >95% in macaques. The significant reduction in serum TTR levels following TTR gene editing indicates that this approach could be an effective treatment for ATTR.
Keywords: AAV; gene editing; gene therapy; meganuclease; transthyretin; transthyretin amyloidosis.
Conflict of interest statement
The authors declare having potential competing financial interests. J.M.W. is a paid advisor to and holds equity in iECURE, Scout Bio, Passage Bio, and the Center for Breakthrough Medicines (CBM). He also holds equity in the G2 Bio-associated asset companies. He has sponsored research agreements with Amicus Therapeutics, Biogen, CBM, Elaaj Bio, FA212, G2 Bio, G2 Bio-associated asset companies, iECURE, Janssen, Passage Bio, and Scout Bio, which are licensees of Penn technology. J.M.W. and J.A.G. are inventors on patents that have been licensed to various biopharmaceutical companies and for which they may receive payments. C.G., W.S., H.L., J.S., G.T., and D.J. are all paid employees of Precision BioSciences.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
