Pharmacological interventions for people with borderline personality disorder
- PMID: 36375174
- PMCID: PMC9662763
- DOI: 10.1002/14651858.CD012956.pub2
Pharmacological interventions for people with borderline personality disorder
Abstract
Background: Among people with a diagnosis of borderline personality disorder (BPD) who are engaged in clinical care, prescription rates of psychotropic medications are high, despite the fact that medication use is off-label as a treatment for BPD. Nevertheless, people with BPD often receive several psychotropic drugs at a time for sustained periods.
Objectives: To assess the effects of pharmacological treatment for people with BPD.
Search methods: For this update, we searched CENTRAL, MEDLINE, Embase, 14 other databases and four trials registers up to February 2022. We contacted researchers working in the field to ask for additional data from published and unpublished trials, and handsearched relevant journals. We did not restrict the search by year of publication, language or type of publication.
Selection criteria: Randomised controlled trials comparing pharmacological treatment to placebo, other pharmacologic treatments or a combination of pharmacologic treatments in people of all ages with a formal diagnosis of BPD. The primary outcomes were BPD symptom severity, self-harm, suicide-related outcomes, and psychosocial functioning. Secondary outcomes were individual BPD symptoms, depression, attrition and adverse events.
Data collection and analysis: At least two review authors independently selected trials, extracted data, assessed risk of bias using Cochrane's risk of bias tool and assessed the certainty of the evidence using the GRADE approach. We performed data analysis using Review Manager 5 and quantified the statistical reliability of the data using Trial Sequential Analysis.
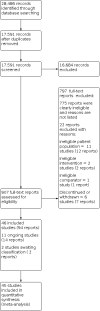
Main results: We included 46 randomised controlled trials (2769 participants) in this review, 45 of which were eligible for quantitative analysis and comprised 2752 participants with BPD in total. This is 18 more trials than the 2010 review on this topic. Participants were predominantly female except for one trial that included men only. The mean age ranged from 16.2 to 39.7 years across the included trials. Twenty-nine different types of medications compared to placebo or other medications were included in the analyses. Seventeen trials were funded or partially funded by the pharmaceutical industry, 10 were funded by universities or research foundations, eight received no funding, and 11 had unclear funding. For all reported effect sizes, negative effect estimates indicate beneficial effects by active medication. Compared with placebo, no difference in effects were observed on any of the primary outcomes at the end of treatment for any medication. Compared with placebo, medication may have little to no effect on BPD symptom severity, although the evidence is of very low certainty (antipsychotics: SMD -0.18, 95% confidence interval (CI) -0.45 to 0.08; 8 trials, 951 participants; antidepressants: SMD -0.27, 95% CI -0.65 to 1.18; 2 trials, 87 participants; mood stabilisers: SMD -0.07, 95% CI -0.43 to 0.57; 4 trials, 265 participants). The evidence is very uncertain about the effect of medication compared with placebo on self-harm, indicating little to no effect (antipsychotics: RR 0.66, 95% CI 0.15 to 2.84; 2 trials, 76 participants; antidepressants: MD 0.45 points on the Overt Aggression Scale-Modified-Self-Injury item (0-5 points), 95% CI -10.55 to 11.45; 1 trial, 20 participants; mood stabilisers: RR 1.08, 95% CI 0.79 to 1.48; 1 trial, 276 participants). The evidence is also very uncertain about the effect of medication compared with placebo on suicide-related outcomes, with little to no effect (antipsychotics: SMD 0.05, 95 % CI -0.18 to 0.29; 7 trials, 854 participants; antidepressants: SMD -0.26, 95% CI -1.62 to 1.09; 2 trials, 45 participants; mood stabilisers: SMD -0.36, 95% CI -1.96 to 1.25; 2 trials, 44 participants). Very low-certainty evidence shows little to no difference between medication and placebo on psychosocial functioning (antipsychotics: SMD -0.16, 95% CI -0.33 to 0.00; 7 trials, 904 participants; antidepressants: SMD -0.25, 95% CI -0.57 to 0.06; 4 trials, 161 participants; mood stabilisers: SMD -0.01, 95% CI -0.28 to 0.26; 2 trials, 214 participants). Low-certainty evidence suggests that antipsychotics may slightly reduce interpersonal problems (SMD -0.21, 95% CI -0.34 to -0.08; 8 trials, 907 participants), and that mood stabilisers may result in a reduction in this outcome (SMD -0.58, 95% CI -1.14 to -0.02; 4 trials, 300 participants). Antidepressants may have little to no effect on interpersonal problems, but the corresponding evidence is very uncertain (SMD -0.07, 95% CI -0.69 to 0.55; 2 trials, 119 participants). The evidence is very uncertain about dropout rates compared with placebo by antipsychotics (RR 1.11, 95% CI 0.89 to 1.38; 13 trials, 1216 participants). Low-certainty evidence suggests there may be no difference in dropout rates between antidepressants (RR 1.07, 95% CI 0.65 to 1.76; 6 trials, 289 participants) and mood stabilisers (RR 0.89, 95% CI 0.69 to 1.15; 9 trials, 530 participants), compared to placebo. Reporting on adverse events was poor and mostly non-standardised. The available evidence on non-serious adverse events was of very low certainty for antipsychotics (RR 1.07, 95% CI 0.90 to 1.29; 5 trials, 814 participants) and mood stabilisers (RR 0.84, 95% CI 0.70 to 1.01; 1 trial, 276 participants). For antidepressants, no data on adverse events were identified.
Authors' conclusions: This review included 18 more trials than the 2010 version, so larger meta-analyses with more statistical power were feasible. We found mostly very low-certainty evidence that medication may result in no difference in any primary outcome. The rest of the secondary outcomes were inconclusive. Very limited data were available for serious adverse events. The review supports the continued understanding that no pharmacological therapy seems effective in specifically treating BPD pathology. More research is needed to understand the underlying pathophysiologic mechanisms of BPD better. Also, more trials including comorbidities such as trauma-related disorders, major depression, substance use disorders, or eating disorders are needed. Additionally, more focus should be put on male and adolescent samples.
Trial registration: ClinicalTrials.gov NCT00254748 NCT00880919 NCT03418675 NCT02097706 NCT00222482 NCT00635921 NCT00634062 NCT01133301 NCT00124839 NCT00091650 NCT00088036 NCT00255554 NCT00463775 NCT00633802 NCT01103180 NCT00437099 NCT01912391 NCT03495375 NCT04100096 NCT04566601.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Jutta M Stoffers‐Winterling (JSW) is a board‐certified clinical psychologist ('Psychologische Psychotherapeutin', cognitive behaviour therapy). She does not prescribe or administer medication in a clinical context.
Ole Jakob Storebø (OJS) is a clinical psychologist with the Psychiatric Research Unit, Region Zealand Psychiatry, Denmark, and does not prescribe or administer medication in a clinical context. He also reports being the trial coordinator for an ongoing study at Region Zealand investigating a new drug treatment for people with borderline personality disorder (NCT04566601), which is included in this review; the study is funded and designed by Boehringer Ingelheim. OJS did not assess the eligibility of this trial which was assessed by two independent reviewers (JPR and JSW). In addition, OJS is an editor for Cochrane Developmental, Psychosocial and Learning Problems (DPLP); however, he was not involved in the editorial process for this review. OJS is also Editor‐in‐Chief for the Scandinavian Journal of Child and Adolescent Psychiatry and Psychology.
Jessica T Mattivi (JTM) is currently working with Psychiatric Services in Meran, Italy. JTM has declared a grant from the German Federal Ministry of Education and Research, paid to University Medical Center Mainz, Germany. The grant was for a systematic review on psychosocial interventions for self‐harm in adolescents; the funder did not have any role in design, methods, data analysis and reporting of the study.
Mickey T Kongerlev is a clinical psychologist and does not prescribe or administer medication in a clinical context. He is employed as manager of a District Psychiatric Service in Region Zealand Mental Health Services, Roskilde, Denmark, and is President of the Institute of Personality Theory and Psychopathology.
Birgit A Völlm is a medical practitioner with the University of Rostock, with the authority to prescribe medication in a clinical context. She is affiliated to the Royal College of Psychiatrists and the DGPPN (Deutsche Gesellschaft für Psychiatrie, Psychotherapie, Psychosomatik und Nervenheilkunde; the German Association for Psychiatry, Psychotherapy and Psychosomatics, which is the largest scientific medical association focusing on mental health in Germany), who have declared an opinion or position on the topic.
Henriette E Callesen is a neuroscientist and does not prescribe or administer medication in a clinical context. She has declared that she has no conflicts of interest.
Adnan Todorovac is a clinical psychologist and does not prescribe or administer medication in a clinical context. He has declared that he has no conflicts of interest.
Christian P Sales is a clinical psychologist with Nottinghamshire Healthcare NHS Foundation Trust, UK, and does not prescribe or administer medication in a clinical context. He has declared that he has no conflicts of interest.
Erlend Faltinsen is a clinical psychologist and does not prescribe or administer medication in a clinical context.
Mie S Jørgensen is a clinical psychologist with Region Zealand Mental Health Services, Denmark, and does not prescribe or administer medication in a clinical context. She has declared that she has no conflicts of interest.
Johanne P Ribeiro is a registered nurse with the authority to administer prescribed medications; however, she is employed as a research assistant at Region Zealand and therefore does not administer medication in a clinical context. She has declared that she has no conflicts of interest.
Julie P Schaug is a psychologist and does not prescribe or administer medication in a clinical context. She has declared that she has no conflicts of interest.
Erik Simonsen (ES) is a psychiatrist with the authority to prescribe medication in a clinical context but does not exercise this authority as he is currently employed as a research director. ES reports that he is the Princpal Investigator of one site (Slagelse, Denmark) of an ongoing, international, multi‐site study investigating a new drug treatment for people with borderline personality disorder (NCT04566601), which is included in this review; the study is funded and designed by Boehringer Ingelheim. ES did not assess the eligibility of this trial, which was assessed by two independent reviewers (JPR and JSW). ES also reports travel and associated expenses (congress fees and accommodation) from Boehringer Ingelheim.
Klaus Lieb (KL) is a board‐certified cognitive behaviour therapist with a special interest in schema therapy. He is also a psychiatrist with the authority to prescribe medication (i.e. recommend treatments for personality disorders); however, he is employed as Director of the Department of Psychiatry and Psychotherapy, University Medical Centre Mainz, Germany and therefore does not prescribe or administer medication in a clinical context. KL is also an editor for DPLP but was not involved in the editorial process for this review.
Figures



















































































































Update of
References
References to studies included in this review
Amminger 2013 {published data only}
-
- Amminger GP, Chanen AM, Ohmann S, Klier CM, Mossaheb N, Bechdolf A, et al. Omega-3 fatty acid supplementation in adolescents with borderline personality disorder and ultra-high risk criteria for psychosis: a post hoc subgroup analysis of a double-blind, randomized controlled trial. Canadian Journal of Psychiatry [Revue Canadienne de Psychiatrie] 2013;58(7):402-8. [DOI: 10.1177/070674371305800705] [PMID: ] - DOI - PubMed
-
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of General Psychiatry 2010;67(2):146-54. [DOI: 10.1001/archgenpsychiatry.2009.192] [PMID: ] - DOI - PubMed
AstraZeneca 2007 {published data only}
-
- NCT00254748. Verkes Borderline Study: the effect of quetiapine on borderline personality disordered patients [The effect of quetiapine on psychotic-like symptoms in borderline personality disordered patients: a randomised placebo-controlled trial]. clinicaltrials.gov/ct2/show/NCT00254748 (first received 15 November 2005).
-
- Van den Broek PJ, Penterman EJ, Hummelen JW, Verkes RJ. P.3.c.038. The effect of quetiapine on psychotic-like symptoms in borderline personality disorder. A placebo-controlled trial. European Neuropsychopharmacology 2008;18(Suppl 4):S425-6. [DOI: 10.1016/S0924-977X(08)70622-9] - DOI
Bellino 2014 {published data only}
-
- Bellino S, Bozzatello P, Brunetti C, De Grandi E, Bogetto F. Omega-3 fatty acids associated with valproate in the treatment of borderline personality disorder: a controlled study [Acidi grassi omega-3 associati alvalproato nel trattamento del disturbo borderlinedi personalità: uno studio controllato]. Journal of Psychopathology 2013;19(4):296-303. [WEB PAGE: Available at www.jpsychopathol.it/wp-content/uploads/2015/07/02-Bellino-acidi1.pdf]
Black 2014 {published data only}
-
- Bellino S. Inclusion of study in Cochrane review. Email to: A Tadorovac 31 January 2019.
-
- Black DW, Zanarini MC, Romine A, Shaw M, Allen J, Schulz SC. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry 2014;171(11):1174-82. [DOI: 10.1176/appi.ajp.2014.13101348] [PMID: ] - DOI - PubMed
-
- Lee SS, Allen J, Black DW, Zanarini MC, Schulz SC. Quetiapine's effect on the SCL-90-R domains in patients with borderline personality disorder. Annals of Clinical Psychiatry 2016;28(1):4-10. [PMID: ] - PubMed
-
- NCT00880919. Seroquel extended release (XR) for the management of borderline personality disorder (BPD) [Seroquel XR for the management of borderline personality disorder (BPD)]. clinicaltrials.gov/ct2/show/NCT00880919 (first received 10 April 2009).
Bogenschutz 2004 {published data only}
-
- Nurnberg HG, Bogenschutz MP. Atypical antipsychotic treatment of BPD: a 12-week, double-blind, placebo-control trial with olanzapine. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Fransisco (CA). 2003.
Bozzatello 2017 {published data only}ACTRN12614000551695
-
- ACTRN12614000551695. Efficacy and tolerability of asenapine compared with olanzapine in borderline personality disorder: a randomized controlled trial [Asenapine versus olanzapine in the treatment of impulsivity, aggression, and self injuries in patients with borderline personality disorder]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365182 (first received 12 May 2014).
Cowdry 1988 {published data only}
-
- Gardner DL, Cowdry RW. Development of melancholia during carbamazepine treatment in borderline personality disorder. Journal of Clinical Psychopharmacology 1986;6(4):236-9. [PMID: ] - PubMed
Crawford 2018 {published data only}
-
- Crawford MJ, Sanatinia R, Barrett B, Byford S, Cunningham G, Gakhal K, et al. Lamotrigine versus inert placebo in the treatment of borderline personality disorder: study protocol for a randomized controlled trial and economic evaluation. Trials 2015;16:308. [DOI: 10.1186/s13063-015-0823-x] [PMCID: PMC4506596] [PMID: ] - DOI - PMC - PubMed
-
- Crawford MJ. Inclusion of your study in Cochrane review. Email to: A Pathmanathan Ratnam 09 November 2020.
De la Fuente 1994 {published data only}
Frankenburg 2002 {published data only}
Goldberg 1986 {published data only}
Grant 2022 {published data only}
Hallahan 2007 {published data only}
Hollander 2001 {published data only}
Jariani 2010 {published data only}
-
- Jariani M, Saaki M, Nazari H, Birjandi M. The effect of olanzapine and sertraline on personality disorder in patients with methadone maintenance therapy. Psychiatria Danubina 2010;22(4):544-7. [PMID: ] - PubMed
Kulkarni 2018 {published data only}
-
- Kulkarni J, Thomas N, Hudaib AR, Gavrilidis E, Grigg J, Tan R, et al. Effect of the glutamate NMDA receptor antagonist memantine as adjunctive treatment in borderline personality disorder: an exploratory, randomised, double-blind, placebo-controlled trial. Central Nervous System Drugs 2018;32(2):179-87. [DOI: 10.1007/s40263-018-0506-8] [PMID: ] - DOI - PubMed
-
- Kulkarni J [pers comm]. Re: Your Request. Email to: A Pathmanatan Ratnam 14 December 2020.
-
- NCT02097706. A novel drug for borderline personality disorder [A randomised double-blind placebo controlled investigation of the efficacy of a novel drug as an adjunct in patients with borderline personality disorder]. clinicaltrials.gov/ct2/show/study/NCT02097706 (first received 25 March 2014).
Leone 1982 {published data only}
-
- Leone NF. Response of borderline patients to loxapine and chlorpromazine. Journal of Clinical Psychiatry 1982;43(4):148-50. [PMID: ] - PubMed
Linehan 2008 {published data only}
-
- Linehan M. Update on dialectical behavioral therapy for BPD. In: 158th Annual Meeting of the American Psychiatric Association; 2005 May 21-26; Atlanta (GA). 2005.
-
- Linehan MM, McDavid JD, Brown MZ, Sayrs JH, Gallop RJ. Olanzapine plus dialectical behavior therapy for women with high irritability who meet criteria for borderline personality disorder: a double-blind, placebo-controlled pilot study. Journal of Clinical Psychiatry 2008;69(9):999-1005. [DOI: 10.4088/jcp.v69n0617] [PMID: ] - DOI - PubMed
Loew 2006 {published data only}
-
- Loew TH, Nickel MK, Muehlbacher M, Kaplan P, Nickel C, Kettler C, et al. Topiramate treatment for women with borderline personality disorder: a double-blind, placebo-controlled study. Journal of Clinical Psychopharmacology 2006;26(1):61-6. [DOI: 10.1097/01.jcp.0000195113.61291.48] [PMID: ] - DOI - PubMed
Markovitz 1995a {published data only}
-
- Markovitz PJ. Pharmacotherapy of impulsivity, aggression, and related disorders. In: Hollander E, Stein DJ, editors(s). Impulsivity and Aggression. New York (NY): Wiley, 1995:263-87.
Moen 2012 {published data only}
-
- Moen R, Freitag M, Miller M, Lee S, Romine A, Song S, et al. Efficacy of extended-release divalproex combined with "condensed" dialectical behavior therapy for individuals with borderline personality disorder. Annals of Clinical Psychiatry 2012;24(4):255-60. [PMID: ] - PubMed
-
- NCT00222482. Depakote ER in borderline personality disorder [A double-blind and placebo controlled assessment of depakote ER in borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT00222482 (first received 14 September 2005).
Montgomery 1982a {published data only}
-
- Montgomery SA, Montgomery DB, Janyanthi Rani S, Roy DH, Shaw PJ, McAuley R. Maintenance therapy in repeat suicidal behaviour: a placebo controlled trial. In: 10th International Congress for Suicide Prevention and Crisis Intervention; 1979 Jun 17-20; Ottawa (ON). 1979.
Montgomery 1982b {published data only}
-
- Montgomery D, Montgomery S, Roy D. Mianserin in the prophylaxis of suicidal behaviour: a double-blind placebo controlled trial. In: Soubrier JP, Vedrinne J, editors(s). Depression and Suicide: Aspects Medicaux, Psychologiques et Socio-Culturels. Paris (FR): Pergamon, 1983:786-90. [DOI: 10.1016/B978-0-08-027080-7.50130-0] - DOI
NCT00533117 {published data only}
-
- Fertuck EA, Keilp J, Song I, Morris MC, Wilson ST, Brodsky BS, et al. Higher executive control and visual memory performance predict treatment completion in borderline personality disorder. Psychotherapy and Psychosomatics 2012;81(1):38-43. [DOI: 10.1159/000329700] [PMCID: PMC3242704] [PMID: ] - DOI - PMC - PubMed
-
- NCT00533117. Treating suicidal behavior and self-mutilation in people with borderline personality disorder [Treating suicidal behavior and self-mutilation in borderline personality disorder]. clinicaltrials.gov/ct2/show/results/NCT00533117?sect=X043716&view=re... (first received 19 September 2007).
Nickel 2004 {published data only}
-
- Nickel MK, Nickel C, Mitterlehner FO, Tritt K, Lahmann C, Leiberich PK, et al. Topiramate treatment of aggression in female borderline personality disorder patients: a double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2004;65(11):1515-9. [DOI: 10.4088/jcp.v65n1112] [PMID: ] - DOI - PubMed
Nickel 2005 {published data only}
Nickel 2006 {published data only}
-
- Nickel MK, Mühlbacher M, Nickel C, Kettler C, Pedrosa Gil F, Bachler E, et al. Aripiprazole in the treatment of patients with borderline personality disorder: a double-blind, placebo-controlled study. American Journal of Psychiatry 2006;163(5):833-8. [DOI: 10.1176/ajp.2006.163.5.833] [PMID: ] - DOI - PubMed
Pascual 2008 {published data only}
-
- NCT00635921. Ziprasidone in the treatment of borderline personality disorder [Ziprasidone in the treatment of borderline personality disorder: a double-blind, placebo-controlled, randomized study]. clinicaltrials.gov/ct2/show/NCT00635921 (accessed 9 September 2009).
Reich 2009 {published data only}
-
- NCT00634062. Study of lamotrigine treatment of affective instability in borderline personality disorder. clinicaltrials.gov/ct2/show/NCT00634062 (first received 4 March 2008). [NCT00634062]
Rinne 2002 {published data only}
-
- Rinne T, Van den Brink W, Wouters L, Van Dyck R. SSRI treatment of borderline personality disorder: a randomized, placebo-controlled clinical trial for female patients with borderline personality disorder. American Journal of Psychiatry 2002;159(12):2048-54. [DOI: 10.1176/appi.ajp.159.12.2048] [PMID: ] - DOI - PubMed
Salzman 1995 {published data only}
Schmahl 2012a {published data only}
-
- NCT01133301. Naltrexone in the treatment of dissociative symptoms in patients with borderline personality disorder. clinicaltrials.gov/ct2/show/NCT01133301 (first received 17 May 2010).
Schmahl 2012b {published data only}
-
- NCT00124839. Naltrexone in borderline personality disorder [Evaluation of the efficacy of the opioid antagonist naltrexone on the incidence and intensity of flashbacks and dissociative states in patients with borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT00124839 (first received 27 July 2005).
-
- NCT01133301. Naltrexone in the treatment of dissociative symptoms in patients with borderline personality disorder. clinicaltrials.gov/ct2/show/record/NCT01133301 (first received 17 May 2010).
Schulz 2007 {published data only}
-
- Eli Lilly and Company. Efficacy and safety of olanzapine in patients with borderline personality disorder: a randomized, flexible-dose, double-blind comparison with placebo [Summary ID #6257. Clinical Study Summary: F1D-MC-HGKL]. www.lillytrials.com/results_files/zyprexa/zyprexa_summary_6257 (accessed 14 February 2008).
-
- NCT00091650. Olanzapine in patients with borderline personality disorder [Efficacy and safety of olanzapine in patients with borderline personality disorder: a randomized flexible dose double-blind comparison with placebo]. clinicaltrials.gov/ct2/show/NCT00091650 (first accessed 14 September 2004).
-
- Schulz SC, Zanarini M, Detke H, Trzaskoma Q, Lin D, DeBerdt W, et al. P02.101 Olanzapine for the treatment of borderline personality disorder: a flexible-dose 12-week randomized double-blind placebo-controlled study. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S191. [DOI: 10.1017/S1461145706007292] - DOI
-
- Schulz SC, Zanarini MC, Bateman A, Bohus M, Detke HC, Trzaskoma Q, et al. Olanzapine for the treatment of borderline personality disorder: variable dose, 12-week randomised double-blind placebo-controlled study. British Journal of Psychiatry 2008;193(6):485-92. [DOI: 10.1192/bjp.bp.107.037903] [PMID: ] - DOI - PubMed
-
- Schulz SC, Zanarini MC, Detke HC, Trzaskoma Q, Lin D, DeBerdt W, et al. Olanzapine for the treatment of borderline personality disorder: a flexible‐dose, 12‐week, randomized, double‐blind, placebo‐controlled study. In: Association of European Psychiatrsts. 15th European Congress of Psychiatry; 2007 Mar 17‐21; Madrid, Spain. 2007.
Shafti 2010 {published data only}
Shafti 2014 {published data only}
-
- Shafti SS, Kaviani H. A comparative study on olanzapine and aripiprazole for symptom management in female patients with borderline personality disorder. Klinik Psikofarmakoloji Bülteni [Bulletin of Clinical Psychopharmacology] 2015;25(1):38-43. [DOI: 10.5455/bcp.20140923100030] - DOI
-
- Shafti SS, Kaviani H. Olanzapine vs aripiprazole in the management of borderline personality disorder. Current Psychopharmacology 2014;3(2):132-6. [DOI: 10.2174/2211556003666140912223626] - DOI
Simpson 2004 {published data only}
Soler 2005 {published data only}
-
- Pascual JC, Soler J, Barrachina J, Campins J, Puigdemont D, Alvarez E, et al. Olanzapine plus dialectical behavior therapy of patients with BPD. In: Psychotherapy and Psychopharmacology: Dissolving the Mind-Brain Barrier. 157th Annual Meeting of the American Psychiatric Association; 2004 May 1-6; New York (NY). 2004.
-
- Soler J, Pascual JC, Campins J, Barrachina J, Puigdemont D, Alvarez E, et al. Double-blind, placebo-controlled study of dialectical behavior therapy plus olanzapine for borderline personality disorder. American Journal of Psychiatry 2005;162(6):1221-4. [DOI: 10.1176/appi.ajp.162.6.1221] [PMID: ] - DOI - PubMed
Soloff 1989 {published data only}
-
- Soloff PH, George A, Nathan RS, Schulz PM, Perel JM. Behavioral dyscontrol in borderline patients treated with amitriptyline. Psychopharmacology Bulletin 1987;23(1):177-81. [PMID: ] - PubMed
-
- Soloff PH, George A, Nathan S, Schulz PM, Ulrich RF, Perel JM. Amitriptyline and haloperidol in unstable and schizotypal borderline disorders. Psychopharmacology Bulletin 1986;22(1):177-82. [PMID: ] - PubMed
Soloff 1993 {published data only}
-
- Cornelius JR, Soloff PH, George A, Ulrich RF, Perel JM. Haloperidol vs phenelzine in continuation therapy of borderline disorder. Psychopharmacology Bulletin 1993;29(2):333-7. [PMID: ] - PubMed
Tritt 2005 {published data only}
Zanarini 2001 {published data only}
Zanarini 2003 {published data only}
Zanarini 2004 {published data only}
Zanarini 2007 {published data only}
-
- Eli Lilly and Company. Efficacy and safety of olanzapine in patients with borderline personality disorder: a randomized double-blind comparison with placebo [Summary ID # 6253. Clinical Study Summary: Study F1D-MC-HGKK]. www.lillytrials.com/results_files/zyprexa/zyprexa_summary_6523/ (accessed 14 February 2008).
-
- NCT00088036. Efficacy and safety of olanzapine in patients with borderline personality disorder [Efficacy and safety of olanzapine in patients with borderline personality disorder: a randomized double-blind comparison with placebo]. clinicaltrials.gov/ct2/show/NCT00088036 (first received 19 July 2004).
-
- Zanarini MC, Schulz SC, Detke HC, Tanaka Y, Zhao F, Lin D, et al. A dose comparison of olanzapine for the treatment of borderline personality disorder: a 12-week randomized, double-blind, placebo controlled study. European Psychiatry 2007;22(Suppl 1):S172-3. [DOI: 10.1016/j.eurpsy.2007.01.565] - DOI - PubMed
-
- Zanarini MC, Schulz SC, Detke HC, Tanaka Y, Zhao F, Lin D, et al. A dose comparison of olanzapine for the treatment of borderline personality disorder: a 12-week randomized, double-blind, placebo-controlled study. In: Association of European Psychiatrists. 15th European Congress of Psychiatry; 2007 Mar 17-21; Madrid, Spain. 2007.
-
- Zanarini MC, Schulz SC, Detke HC, Tanaka Y, Zhao F, Lin D, et al. P02.102 A dose comparison of olanzapine for the treatment of borderline personality disorder: a 12-week randomized double-blind placebo-controlled study. International Journal of Neuropsychopharmacology 2006;9(Suppl 1):S191. [DOI: 10.1017/S1461145706007450] - DOI - PubMed
Ziegenhorn 2009 {published data only}
-
- Ziegenhorn AA, Roepke S, Schommer NC, Merkl A, Danker-Hopfe H, Perschel FH, et al. Clonidine improves hyperarousal in borderline personality disorder with or without comorbid posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology 2009;29(2):170-3. [DOI: 10.1097/JCP.0b013e31819a4bae] [PMID: ] - DOI - PubMed
References to studies excluded from this review
ACTRN12615000705583 {published data only}
-
- ACTRN12615000705583. Investigation of the benefits of using quetiapine as an adjunct treatment in addition to psychotherapy in patients diagnosed with severe Borderline Personality Disorder. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368589 (first received 8 July 2015).
Bellino 2006b {published data only}
Coccaro 1997 {published data only (unpublished sought but not used)}
Hollander 2005 {published data only}
ISRCTN11135486 {published data only}11135486
-
- ISRCTN11135486. Quetiapine versus sertraline as the pharmacological component in a standardised psychopharmacological and psychotherapeutic treatment of borderline personality disorder: a randomised, rater-blinded study. www.isrctn.com/ISRCTN11135486 (first received 1 August 2006). [ISRCTN11135486]
-
- Malevani J. AW: Cochrane review: RCT zu Quetapin und Sertraline bei BPS [personal communication]. Email to: J Stoffers-Winterling 17 June 2020.
Koenigsberg 2003 {published data only}
La Malfa 2003 {published data only}
-
- La Malfa G, Lampronti L, Bertelli M, Conte M, Cabras P. Aggressiveness and personality disorders: evaluation of efficacy and existence of predictors in the treatment with fluvoxamine and lithium [Aggressività e disturbi della personalità]. Minerva Psychiatrica 2003;44(2):75-82. [WEB PAGE: Available at www.minervamedica.it/en/journals/minerva-psychiatry/article.php?cod=R17Y...
Links 1990 {published data only}
-
- Links PS, Steiner M, Boiago I, Irwin D. Lithium therapy for borderline patients: preliminary findings. Journal of Personality Disorders 1990;4(2):173-81. [DOI: 10.1521/pedi.1990.4.2.173] - DOI
Marchesi 2006 {published data only}
-
- Marchesi C, De Panfilis C, Cantoni A, Fontò S, Giannelli MR, Maggini C. Personality disorders and response to medication treatment in panic disorder: a 1-year naturalistic study. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2006;30(7):1240-5. [DOI: 10.1016/j.pnpbp.2006.03.010] [PMID: ] - DOI - PubMed
NCT00255554 {published data only}
-
- Goodman M, New AS, Koenigsberg HW, Hazlett E, Flory J, Siever L. Psychotherapy and combined treatment in BPD: possible effects. In: Psychosomatic Medicine. Integrating Psychiatry & Medicine. 158th Annual Meeting of the American Psychiatric Association; 2005 May 21-26; Atlanta (GA). 2005:126-7. [ABSTRACT #: No. 19F] [PDF: www.psychiatry.org/File%20Library/Psychiatrists/Directories/Library-and-...
-
- Goodman M. RE: [EXTERNAL] Cochrane Colllaboration review [personal communication]. Email to: J Stoffers-Winterling 16 June 2018.
-
- NCT00255554. DBT and escitalopram in borderline personality disorder [Effects of dialectical behavioral therapy and escitalopram on impulsive aggression, affective instability and cognitive processing in borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT00255554 (first received 17 November 2005). [NCT00255554]
NCT00463775 {published data only}
-
- NCT00463775. Topiramate for treatment of patients with borderline personality disorder and alcohol dependence. clinicaltrials.gov/ct2/show/NCT00463775 (first received 18 April 2007).
NCT00633802 {published data only}
-
- Bloch M. Re: RCT of Risperidone in borderline PD [pers commun]. Email to: J. Stoffers-Winterling 15 August 2018.
-
- NCT00633802. Low-dose risperidone treatment for subjects suffering from borderline personality disorder. clinicaltrials.gov/ct2/show/NCT00633802 (first received 4 March 2008).
NCT01103180 {published data only}
-
- McCloskey M. Fwd: Cochrane Collaboration review - RCT of escitalopram in BPD. Email to: J Stoffers-Winterling 15 August 2018.
-
- NCT01103180. Selective serotonin reuptake inhibitors (SSRIs) in borderline personality disorder [SSRIs and self-harm in borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT01103180 (first received 12 April 2010).
NCT03395314 {published data only}
-
- NCT03395314. Ketamine in borderline personality disorder [A randomized active placebo controlled trial of ketamine in borderline personality disorderKetamine in Borderline Personality Disorder]. clinicaltrials.gov/ct2/show/NCT03395314 (first received 10 January 2018). [NCT03395314]
Parsons 1989 {published data only}
-
- Parsons B, Quitkin FM, McGrath PJ, Stewart JW, Tricamo E, Ocepek-Welikson K, et al. Phenelzine, imipramine, and placebo in borderline patients meeting criteria for atypical depression. Psychopharmacology Bulletin 1989;25(4):524-34. [PMID: ] - PubMed
Rombold 2014 {published data only}
-
- Rombold F, Lauterbach E, Felber W, Mueller-Oerlinghausen B, Ahrens B, Bronisch T, et al. Adjunctive lithium treatment in the prevention of suicidal behavior in patients with depression and comorbid personality disorders. International Journal of Psychiatry in Clinical Practice 2014;18(4):300-3. [DOI: 10.3109/13651501.2014.940052] [PMID: ] - DOI - PubMed
Russell 2003 {published data only}
Serban 1984 {published data only}
Verkes 1998 {published data only}
Wollmer 2022 {published data only}
-
- Wollmer MA, Neumann I, Jung S, Bechinie A, Herrmann J, Müller A, et al. Clinical effects of glabellar botulinum toxin injections on borderline personality disorder: a randomized controlled trial. Journal of Psychopharmacology 2022;36(2):159-69. [DOI: 10.1177/02698811211069108] [PMID: ] - DOI - PubMed
References to studies awaiting assessment
NCT00437099 {published data only}
-
- NCT00437009. Efficacy of omega-3 fatty acids on borderline personality disorder: a randomised, double blind clinical trial. clinicaltrials.gov/ct2/show/NCT00437099 (first received 16 February 2007).
NCT01912391 {published data only}
-
- NCT01912391. Clinical research study to evaluate selegiline in the treatment of borderline personality disorder [A phase III randomized double-blind, 12 week, placebo controlled trial of transdermal selegiline in borderline personality disorder (BPD) to evaluate efficacy and safety]. clinicaltrials.gov/ct2/show/NCT01912391 (first received 16 July 2013).
References to ongoing studies
ACTRN12617001317381 {published data only}ACTRN12617001317381
-
- ACTRN12617001317381. Estrogen for the treatment of borderline personality disorder [A randomised placebo controlled trial of estradiol for the treatment of women with borderline personality disorder]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373455 (first received 10 August 2017).
Arteaga‐Henríquez 2020 {published data only}
-
- Arteaga-Henríquez G, Rosales-Ortiz SK, Arias-Vásquez A, Bitter I, Ginsberg Y, Ibañez-Jimenez P, et al. Treating impulsivity with probiotics in adults (PROBIA): study protocol of a multicenter, double-blind, randomized, placebo-controlled trial. Trials 2020;21(1):161. [DOI: 10.1186/s13063-019-4040-x] [PMCID: PMC7014653] [PMID: ] - DOI - PMC - PubMed
-
- NCT03495375. Treating impulsivity in adults with probiotics (PROBIA) [Randomized placebo-controlled treatment of impulsivity in adults with probiotics]. clinicaltrials.gov/ct2/show/NCT03495375 (first received 26 February 2018).
Chanen 2019 {published data only}ACTRN12616001192471
-
- ACTRN12616001192471. VERBATIM: a randomised controlled trial of aripiprazole for the treatment of auditory verbal hallucinations in borderline personality disorder [A randomised controlled trial of aripiprazole for the treatment of auditory verbal hallucinations in borderline personality disorder]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371038 (first received 26 August 2016).
-
- Chanen AM, Betts J, Jackson H, McGorry P, Nelson B, Cotton SM, et al. Ripiprazole compared with placebo for auditory verbal hallucinations in youth with borderline personality disorder: protocol for the VERBATIM randomized controlled trial. Early Intervention in Psychiatry 2019;13(6):1373-81. [DOI: 10.1111/eip.12774] [PMID: ] - DOI - PubMed
DRKS00015817 {published data only}
-
- DRKS00015817. Stellate ganglion block in patients with borderline personality disorder and posttraumatic stress disorder. www.drks.de/DRKS00015817 (first received 31 October 2018).
EUCTR2020‐003469‐20‐ES {published data only}
-
- EUCTR2020-003469-20-ES. A phase IIb study to evaluate the safety and efficacy of vafidemstat in an adult borderline personality disorder population [A double blind, randomized, placebo-controlled, adaptive 14-week phase IIb trial to evaluate the efficacy and safety of vafidemstat in an adult borderline personality disorder (BPD) population (PORTICO)]. trialsearch.who.int/?TrialID=EUCTR2020-003469-20-ES (first received 19 November 2020). [EUCTR2020-003469-20-ES]
EudraCT 2018‐002471‐18‐GB {published data only}ISRCTN18352058
-
- EudraCT 2018-002471-18. Clozapine in the treatment of borderline personality disorder [The clinical effectiveness and cost effectiveness of clozapine for inpatients with borderline personality disorder: randomised controlled trial]. clinicaltrialsregister.eu/ctr-search/search?query=2018-002471-18/GB (first received 19 June 2019).
-
- ISRCTN18352058. Clozapine in the treatment of borderline personality disorder. The CALMED study [The clinical effectiveness and cost effectiveness of clozapine for inpatients with borderline personality disorder: randomised controlled trial]. www.isrctn.com/ISRCTN18352058 (first received 18 March 2019).
IRCT20210106049948N1 {published data only}IRCT20210106049948N1
-
- IRCT20210106049948N1. Memantine and borderline personality disorder [The effect of memantine on the symptoms of Borderline personality disorder in the Iranian population]. trialsearch.who.int/?TrialID=IRCT20210106049948N1 (first received 21 February 2021). [IRCT20210106049948N1]
IRCT20210531051453N1 {published data only}IRCT20210531051453N1
-
- IRCT20210531051453N1. Effect of omega 3 fatty acid in borderline personality disorder [Study the effectiveness of omega 3 fatty acid as adjuvant treatment on depression, aggression and poor impuls control in hospitalized patients of borderline personality disorder]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20210531051453N1 (first received 30 July 2021). [IRCT20210531051453N1]
NCT04100096 {published data only}
-
- NCT04100096. A trial of brexpiprazole in the treatment of borderline personality disorder [A multicenter, randomized, flexible-dose, double-blind trial of brexpiprazole versus placebo for the treatment of adults with borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT04100096 (first received 20 September 2019).
NCT04566601 {published data only}
-
- NCT04566601. A study to test different doses of BI 1358894 and find out whether they reduce symptoms in people with borderline personality disorder [A phase II randomized, double-blinded, placebo-controlled parallel group trial to examine the efficacy and safety of 4 oral doses of BI 1358894 once daily over 12 week treatment period in patients with borderline personality disorder]. clinicaltrials.gov/ct2/show/NCT04566601 (first received 28 September 2020).
NCT05356013 {published data only}
-
- NCT05356013. Caplyta in borderline personality disorder. clinicaltrials.gov/ct2/show/NCT05356013 (first received 02 May 2022).
Additional references
Aguglia 2019
-
- Aguglia A, Serafini G, Nebbia J, Salvi V, Martinotti G, Corbo M, et al. Off-label use of second-generation antipsychotics in borderline personality disorder: a survey of Italian psychiatrists. Journal of Personality Disorders 2021;35(3):321-5. - PubMed
Alvarez‐Tomas 2017
-
- Alvarez-Tomás I, Soler J, Bados A, Martín-Blanco A, Elices M, Carmona C, et al. Long-term course of borderline personality disorder: a prospective 10-year follow-up study. Journal of Personality Disorders 2017;31(5):590-605. - PubMed
Alvarez‐Tomas 2019
Amminger 2010
-
- Amminger GP, Schäfer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Archives of General Psychiatry 2010;67(2):146-54. [DOI: 10.1001/archgenpsychiatry.2009.192] [PMID: ] - DOI - PubMed
Andrews 2013a
-
- Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719–25. [DOI: 10.1016/j.jclinepi.2012.03.013] [PMID: ] - DOI - PubMed
Andrews 2013b
-
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation - determinants of a recommendation’s direction and strength. Journal of Clinical Epidemiology 2013;66(7):726–35. [DOI: 10.1016/j.jclinepi.2013.02.003] [PMID: ] - DOI - PubMed
Ansell 2007
-
- Ansell EB, Sanislow CA, McGlashan TH, Grilo CM. Psychosocial impairment and treatment utilization by patients with borderline personality disorder, other personality disorders, mood and anxiety disorders, and a healthy comparison group. Comprehensive Psychiatry 2007;48(4):329–36. [DOI: 10.1016/j.comppsych.2007.02.001] [PMID: ] - DOI - PubMed
APA 1980
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III). 3rd edition. Washington (DC): American Psychiatric Association, 1980. [ISBN: 978-0-89042-0416]
APA 1987
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R). 3rd edition. Washington (DC): American Psychiatric Association, 1987. [ISBN: 978-0-89042-0195]
APA 1994
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). 4th edition. Washington (DC): American Psychiatric Association, 1994. [ISBN: 978-0-89042-0610]
APA 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR). 4th edition. Washington (DC): American Psychiatric Association, 2000. [ISBN: 978-0-89042-0256]
APA 2001
-
- American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. American Journal of Psychiatry 2001;158(10 Suppl):1-52. [PMID: ] - PubMed
APA 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5 edition. Washington (DC): American Psychiatric Association, 2013. [ISBN: 978-0-89042-554-1]
Arntz 2003
-
- Arntz A, Van den Hoorn M, Cornelis J, Verheul R, Van den Bosch W, De Bie AJHT. Reliability and validity of the borderline personality disorder severity index. Journal of Personality Disorders 2003;17(1):45-59. [PMID: ] - PubMed
Atkins 2004
Ayodeji 2015
Balshem 2011
Barrett 1995
-
- Barrett ES, Stanford MS. Impulsiveness. In: Costello CG, editors(s). Personality Characteristics of the Personality Disordered. New York (NY): Wiley, 1995:91-118. [ISBN: 978-0-471-01529-1]
Bateman 2015
Beck 1961
-
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561-71. [PMID: ] - PubMed
Beck 1979
-
- Beck AT, Kovacs M, Weissman A. The assessment of suicidal intention: the Scale for Suicide Ideation. Journal of Consulting and Clinical Psychology 1979;47(2):343-52. [PMID: ] - PubMed
Bellino 2019 [pers comm]
-
- Bellino S. Inclusion of study in Cochrane review. Email to: A Tadorovac 31 January 2019.
Bender 2001
Bender 2006
Berkey 1996
-
- Berkey CS, Mosteller F, Lau J, Antman E. Uncertainty of the time of first significance in random effects cumulative meta-analysis. Controlled Clinical Trials 1996;17(5):357-71. [PMID: ] - PubMed
Bernstein 1986
-
- Bernstein EM, Putnam FW. Development, reliability, and validity of a dissociation scale. Journal of Nervous and Mental Disease 1986;174(12):727-35. [PMID: ] - PubMed
Bero 2013
Biskin 2015
Black 2014a
-
- Black DW, Zanarini MC, Romine A, Shaw M, Allen J, Schulz SC. Comparison of low and moderate dosages of extended-release quetiapine in borderline personality disorder: a randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry 2014;171(11):1174-82. [DOI: 10.1176/appi.ajp.2014.13101348] [PMID: ] - DOI - PubMed
Bode 2017
-
- Bode K, Vogel R, Walker J, Kröger C. Health care costs of borderline personality disorder and matched controls with major depressive disorder: a comparative study based on anonymized claims data. European Journal of Health Economics 2017;18(9):1125-35. - PubMed
Bohus 2021
-
- Bohus M, Stoffers-Winterling JM, Sharp C, Krause-Utz A, Schmahl C, Lieb K. Borderline personality disorder. Lancet 2021;398:1528-40. - PubMed
Borschmann 2012
Bridler 2015
-
- Bridler R, Häberle A, Müller ST, Cattapan K, Grohmann R, Toto S, et al. Psychopharmacological treatment of 2195 in-patients with borderline personality disorder: a comparison with other psychiatric disorders. European Neuropsychopharmacology 2015;25(6):763-72. [PMID: 10.1016/j.euroneuro.2015.03.017] [PMID: ] - DOI - PubMed
Brok 2008
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta-analysis may be inconclusive -- trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. International Journal of Epidemiology 2009;38(1):287-98. [DOI: 10.1093/ije/dyn188] [PMID: ] - DOI - PubMed
Brühl 2017
-
- Brühl AB, Sahakian BJ. Neuroscience-based nomenclature: improving clinical and scientific terminology in research and clinical psychopharmacology. Psychological Medicine 2017;47(8):1339-41. - PubMed
Brunetti 2013
Cailhol 2015
-
- Cailhol L, Thalamas C, Garrido C, Birmes P, Lapeyre-Mestre M. Mental health service utilization among borderline personality disorder inpatients [Utilisation des services de soin par les patients hospitalisés, présentant un trouble de personnalité borderline en Midi-Pyrénées]. L'Encéphale 2015;41(2):115-22. [DOI: 10.1016/j.encep.2014.10.008] [PMID: ] - DOI - PubMed
Cavelti 2019
Cavelti 2021
-
- Cavelti M, Thompson K, Chanen AM, Kaess M. Psychotic symptoms in borderline personality disorder: developmental aspects. Current Opinion in Psychology 2021;37:26-31. [PMID: ] - PubMed
Chanen 2012
Chanen 2013
-
- Chanen AM, McCutcheon L. Prevention and early intervention for borderline personality disorder: current status and recent evidence. British Journal of Psychiatry 2013;Supplement(54):s24-9. - PubMed
Chanen 2017
Coccaro 1991
-
- Coccaro EF, Harvey PD, Kupsaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically-based behavioral assessments of impulsive aggressive behavior. Journal of Neuropsychiatry and Clinical Neurosciences 1991;3(2):S44-51. [PMID: ] - PubMed
Coccaro 2020
Coid 2006
Crawford 2020 [pers comm]
-
- Crawford M. Inclusion of your study in Cochrane review. Email to: A Pathmanathan Ratnam 09 November 2020.
Cristea 2017
CTU 2011
-
- Copenhagen Trial Unit. TSA - Trial Sequential Analysis. www.ctu.dk/tsa (accessed 12 December 2016).
Curtin 2002
Deeks 2021
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021), Cochrane 2021. Available from www.training.cochrane.org/handbook.
Derogatis 1994
-
- Derogatis LR, Lazarus L. SCL-90-R, Brief Symptom Inventory, and matching clinical rating scales. In: Maruish ME, editors(s). The Use of Psychological Testing for Treatment Planning and Outcome Assessment. 1st edition. Hillsdale (NJ): Lawrence Erlbaum Associates, 1994. [ISBN: 978-0-80581-1629]
DGPPN 2009
-
- Deutsche Gesellschaft für Psychiatrie und Psychotherapie Psychosomatik und Nervenheilkunde (DGPPN) [German Association for Psychiatry, Psychotherapy and Psychosomatics]. S2-Praxisleitlinien in Psychiatrie und Psychotherapie. Band 1: Behandlungsleitlinie Persönlichkeitsstörungen [S2 Practical Guidelines in Psychiatry and Psychotherapy. Treatment Guideline Personality Disorders]. Vol. 1. Heidelberg (DE): Steinkopff Verlag, 2009. [ISBN: 978-3-7985-1853-7]
Donner 2002
Eaton 2018
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Eli Lilly 2008
-
- Eli Lilly and Company. Efficacy and safety of olanzapine in patients with borderline personality disorder: a randomized, flexible-dose, double-blind comparison with placebo [Summary ID #6257. Clinical Study Summary: F1D-MC-HGKL]. www.lillytrials.com/results_files/zyprexa/zyprexa_summary_6257 (accessed 14 February 2008).
Ellison 2018
Endicott 1976
-
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766-71. [PMID: ] - PubMed
Fonagy 2009
Garner 2016
Gartlehner 2021a
-
- Gartlehner G, Crotty K, Kennedy S, Edlund MJ, Ali R, Siddiqui M, et al. Pharmacological treatments for borderline personality disorder: a systematic review and meta-analysis. Central Nervous System Drugs 2021;35(10):1053-67. [CENTRAL: PMC8478737] [DOI: 10.1007/s40263-021-00855-4] [PMID: ] - DOI - PMC - PubMed
Gartlehner 2021b
-
- Gartlehner G, Crotty K, Edlund MJ, Viswanathan M. Authors’ reply to Pereira Ribeiro et al: comment on “Pharmacological Treatments for Borderline Personality Disorder: A Systematic Review and Meta‑Analysis”. Central Nervous System Drugs 2021;35:1335-6. [DOI: ] - PubMed
Goodman 2012
GRADEpro 2021 [Computer program]
-
- GRADEpro GDT (guideline development tool). Version accessed prior to 18 October 2022. Hamilton (Ontario): Grade Working Group, McMaster University and Evidence Prime, 2021.
Grant 2008
-
- Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry 2008;69:533-45. - PMC - PubMed
Gratz 2001
-
- Gratz KL. Meausrement of deliberate self-harm: preliminary data on the deliberate self-harm inventory. Journal of Psychopathology and Behavioral Assessment 2001;23(4):253-63. [DOI: 10.1023/A:1012779403943] - DOI
Gunderson 2011a
Gunderson 2011b
-
- Gunderson JG, Stout RL, McGlashan TH, Shea MT, Morey LC, Grilo CM, et al. Ten-year course of borderline personality disorder: psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Archives of General Psychiatry 2011;68(8):827-37. [DOI: 10.1001/archgenpsychiatry.2011.37] [PMCID: PMC3158489] [PMID: ] - DOI - PMC - PubMed
Guttierez 2001
Guyatt 2011a
Guyatt 2011b
Guyatt 2011c
Guyatt 2011d
Guyatt 2011e
Guyatt 2011f
Guyatt 2011g
Guyatt 2011h
Guyatt 2013a
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151–7. [DOI: 10.1016/j.jclinepi.2012.01.006] [PMID: ] - DOI - PubMed
Guyatt 2013b
Guyatt 2013c
Gøtzsche 2015
-
- Gøtzsche P. Re: Why the Cochrane risk of bias tool should include funding source as a standard item. Response to JAC Sterne. cochranelibrary.com/editorial/10.1002/14651858.ED000076 (accessed 19 January 2018).
Hancock‐Johnson 2017
Hastrup 2019a
-
- Hastrup LH, Kongerslev MT, Simonsen E. Low vocational outcome among people diagnosed with borderline personality disorder during first admission to mental health services in Denmark: a nationwide 9-year register-based study. Journal of Personality Disorders 2019;33(3):326-40. [DOI: 10.1521/pedi_2018_32_344] - DOI - PubMed
Hastrup 2019b
Herpertz 2007
-
- Herpertz SC, Zanarini M, Schulz CS, Siever L, Lieb K, Möller H-J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of personality disorders. World Journal of Biological Psychiatry 2007;8(4):212-44. [DOI: 10.1080/15622970701685224] [PMID: ] - DOI - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JPT, Altmand DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston M, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [WEB PAGE: Available from www.training.cochrane.org/handbook]
Higgins 2022
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook.
Horowitz 1988
-
- Horowitz LM, Rosenberg SE, Baer BA, Ureño G, Villaseñor VS. Inventory of interpersonal problems: psychometric properties and clinical applications. Journal of Consulting and Clinical Psychology 1988;56(6):885-92. [PMID: ] - PubMed
Hörz 2010
-
- Hörz S, Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. Ten-year use of mental health services by patients with borderline personality disorder and with other axis II disorders. Psychiatric Services 2010;61(6):612-6. [DOI: 10.1176/ps.2010.61.6.612] [PMCID: PMC3889171] [PMID: ] - DOI - PMC - PubMed
Ingenhoven 2011
-
- Ingenhoven TJ, Duivendvoorden HJ. Differential effectiveness of antipsychotics in borderline personality disorder: Meta-analyses of placebo-controlled, randomized clinical trials on symptomatic outcome domains. Journal of Clinical Psychopharmacology 2011;31(4):489-96. [DOI: 10.1097/JCP.0b013e3182217a69] [PMID: ] - DOI - PubMed
Ingenhoven 2015
Jacobi 2021
-
- Jacobi F, Grafiadeli R, Volkmann H, Schneider I. Disease burden of borderline personality disorder: cost of illness, somatic comorbidity and mortality [[Krankheitslast der Borderline-Persönlichkeitsstörung: Krankheitskosten, somatische Komorbidität und Mortalität]]. Nervenarzt 2021;92(7):660-9. - PubMed
Jakobsen 2014
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987;13(2):261-76. [PMID: ] - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Annals of Internal Medicine 2001;135(11):982-9. [PMID: ] - PubMed
Knappich 2014
-
- Knappich M, Hörz-Sagstetter S, Schwerthöffer D, Leucht S, Rentrop M. Pharmacotherapy in the treatment of patients with borderline personality disorder: results of a survey among psychiatrists in private practices. International Clinical Psychopharmacology 2014;29(4):224-8. [DOI: 10.1097/YIC.0000000000000021] [PMCID: PMC4047315] [PMID: ] - DOI - PMC - PubMed
Kongerslev 2015
-
- Kongerslev MT, Chanen AM, Simonsen E. Personality disorder in childhood and adolescence comes of age: a review of the current evidence and prospects for future research. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology 2015;3(1):31-48. [DOI: 10.21307/sjcapp-2015-004] - DOI
Korang 2020
-
- Korang Steven Kwasi, Juul Sophie, Nielsen Emil Eik, Feinberg Joshua, Siddiqui Faiza, Ong Giok, et al. Vaccines to prevent COVID-19: a protocol for a living systematic review with network meta-analysis including individual patient data (The LIVING VACCINE Project). Systematic Reviews 2020;9(1):1-15. - PMC - PubMed
Kulkarni 2020 [pers comm]
-
- Kulkarni J. Re: Your Request. Email to: A Pathmanathan Ratnam 14 December 2020.
Lau 1995
-
- Lau J, Schmid CH, Chalmers TC. Cumulative meta-analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):45-57. [PMID: ] - PubMed
Lenzenweger 2007
Levine 1986
-
- Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology Bulletin 1986;22(2):343-81. [PMID: ] - PubMed
Lieb 2004
Linehan 1997
-
- Linehan MM. Behavioral treatments of suicidal behaviors: definitional obfuscation and treatment outcomes. Annals of the New York Academy of Sciences 1997;836:302-28. [PMID: ] - PubMed
Links 2009
-
- Links PS, Kolla N. Assessing and managing suicide risk. In: Oldham JM, Skodol AE, Bender DS, editors(s). Essentials of Personality Disorders. 1st edition. Arlington (VA): American Psychiatric Publishing, Inc, 2009:343-60. [ISBN: 978-1-58562-358-7]
Lundh 2017
Lundh 2018
-
- Lundh Andreas, Lexchin Joel, Mintzes Barbara, Schroll Jeppe B, Bero Lisa. Industry sponsorship and research outcome: systematic review with meta-analysis. Intensive care medicine 2018;44(10):1603-1612. - PubMed
Makela 2006
Mancke 2015
Meuldijk 2017
Moher 1998
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354(9193):1896-900. [PMID: ] - PubMed
Montgomery 1979
Montgomery 1983
-
- Montgomery D, Montgomery S, Roy D. Mianserin in the prophylaxis of suicidal behaviour: a double‐blind placebo controlled trial. In: Soubrier JP, Vedrinne J, editors(s). Dépression et Suicide: Aspects Medicaux, Psychologiques et Socio-Culturels. Paris (FR): Pergamon Press, 1983:786‐90. [DOI: 10.1016/B978-0-08-027080-7.50130-0] - DOI
Mulder 2021
Munk‐Jørgensen 2010
Mustafa 2013
NbN3 2021
-
- Neuroscience-Based Nomenclature. Neuroscience-Based Nomenclature, Second Edition-reivsed. https://nbn2r.com/search (accessed 02 December 2021).
NCT00880919
-
- NCT00880919. Seroquel extended release (XR) for the management of borderline personality disorder (BPD) [Seroquel XR for the management of borderline personality disorder (BPD)]. clinicaltrials.gov/ct2/show/NCT00880919 (first received 10 April 2009).
Neacsiu 2017
-
- Neacsiu DA, Eberle WJ, Keng SL, Fang MC, Rosenthal ZM. Understanding borderline personality disorder across sociocultural groups: findings, issues, and future directions. Current Psychiatry Reviews 2017;13(3):188-223. [PMID: 10.2174/1573400513666170612122034] - DOI
Newton‐Howes 2021
-
- Newton-Howes G, Cunningham R, Atkinson J. Personality disorder prevalence and correlates in a whole of nation dataset. Soc Psychiatry Psychiatr Epidemiol 2021;56(4):679-85. - PubMed
Ng 2016
NHMRC 2013
-
- National Health and Medical Research Council. Clinical Practice Guideline for the Management of Borderline Personality Disorder. Melbourne: National Health and Medical Research Council, 2013. [WEB PAGE: Available from www.nhmrc.gov.au/guidelines/publications/mh25]
NICE 2009
-
- National Institute for Health and Clinical Excellence. Borderline personality disorder: recognition and management. NICE clinical guideline 78; 28 January 2009. Available at www.nice.org.uk/guidance/cg78 (accessed 29 June 2021).
NICE 2018
-
- National Instiute for Health and Care Excellence (NICE). 2018 surveillance of personality disorders (NICE guidelines CG77 and CG78). Surveillance report. Published 19 July 2018. Available from www.nice.org.uk/guidance/cg78/resources/2018-surveillance-of-personality.... (accessed 05 December 2021). - PubMed
Niesten 2016
Norman 2004
Ose 2021
Osman 2001
Ottosson 2002
Oud 2017
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10(3):799-812. [DOI: 10.2466/pr0.1962.10.3.799] - DOI
Paris 2015
Pascual 2021
-
- Pascual JC, Martín-Blanco A, Soler J. Twenty-Year Trends in the Psychopharmacological Treatment of Outpatients with Borderline Personality Disorder: A Cross-Sectional Naturalistic Study in Spain.. CNS Drugs 2021;35(9):1023-32. - PubMed
Paton 2015
Pereira Ribeiro 2021
-
- Pereira Ribeiro, J, Sedoc Jørgensen, M, Storebø, OJ. Comment on: “Pharmacological Treatments for Borderline Personality Disorder: A Systematic Review and Meta‑Analysis”. CNS Drugs 2021 Nov;35:1333-1334. [https://doi.org/10.1007/s40263-021-00872-3] - PubMed
Perez 2007
-
- Perez V, Barrachina J, Soler J, Pascual JC, Campins MJ, Puigdemont D, et al. The clinical global impression scale for borderline personality disorder patients (CGI-BPD): a scale sensible to detect changes [Impresión clínica global para pacientes con trastorno límite de la personalidad (ICG-TLP): una escala sensible al cambio]. Actas Españolas de Psiquiatría 2007;35(4):229-35. [PMID: ] - PubMed
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 3.2.1. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Riffer 2019
-
- Riffer F, Farkas M, Streibl L, Kaiser E, Sprung M. Psychopharmacological treatment of patients with borderline personality disorder: comparing data from routine clinical care with recommended guidelines. International Journal of Psychiatry in Clinical Practice 2019;23(3):178-88. [DOI: 10.1080/13651501.2019.1576904] [PMID: ] - DOI - PubMed
Rossouw 2012
Sansone 2003
Savović 2012a
-
- Savović J, Jones H, Altman D, Harris R, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assessment 2012;16(35):1-82. [DOI: 10.3310/hta16350] [PMID: ] - DOI - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429-38. [DOI: 10.7326/0003-4819-157-6-201209180-00537] [PMID: ] - DOI - PubMed
Schneider 2019
Schulz 1995
Schulz 2008
-
- Schulz SC, Zanarini MC, Bateman A, Bohus M, Detke HC, Trzaskoma Q, et al. Olanzapine for the treatment of borderline personality disorder: variable dose, 12‐week, randomised double‐blind placebo‐controlled study. British Journal of Psychiatry 2008;193(6):485‐92. [DOI: 10.1192/bjp.bp.107.037903] [PMID: ] - DOI - PubMed
Shea 2017
-
- Shea Beverley J, Reeves Barnaby C, Wells George, Thuku Micere, Hamel Candyce, Moran Julian, Moher David, Tugwell Peter, Welch Vivian, Kristjansson Elizabeth. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. bmj 2017;358:j4008. - PMC - PubMed
Sher 2019
-
- Sher L, Rutter SB, New AS, Siever LJ, Hazlett EA. Gender differences and similarities in aggression, suicidal behaviour, and psychiatric comorbidity in borderline personality disorder. Acta Psychiatr Scand 2019;139(2):145-53. - PubMed
Silk 2015
Simonsen 2019
Skodol 2002
-
- Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. American Journal of Psychiatry 2002;159(2):276-83. [DOI: 10.1176/appi.ajp.159.2.276] [PMID: ] - DOI - PubMed
Soetmann 2008a
-
- Soeteman DI, Hakkaart-van Roijen L, Verheul R, Busschbach JJ. The economic burden of personality disorders in mental health care. Journal of Clinical Psychiatry 2008;69(2):259–65. [PMID: ] - PubMed
Soetmann 2008b
Soloff 2002
Soloff 2012
Spielberger 1988
-
- Spielberger CD. Manual for the State-Trait Anger Expression Inventory. Odessa (Fl): Psychological Assessment Resources, 1988.
Stepp 2012
Sterne 2013
Sterne 2017
-
- Sterne JAC, Egger M, Moger D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Stiglmayr 2005
Stoffers 2015
Stoffers‐Winterling 2018
Stoffers‐Winterling 2020
Stoffers‐Winterling J 2018 [pers comm]
-
- Stoffers-Winterling J. Cochrane Collaboration review - RCT of escitalopram in BPD. Email to: E Coccaro 13 August 2018.
Stone 1993
Storebø 2014
Storebø 2018
Storebø 2020
-
- Storebø OJ, Stoffers-Winterling JM, Völm BA, Kongerslev MT, Mattivi JT, Jørgensen MS, et al. Psychological therapies for people with borderline personality disorder. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD012955. [DOI: 10.1002/14651858.CD012955.pub2] [PMCID: PMC7199382] [PMID: ] - DOI - PMC - PubMed
Ten Have 2016
-
- Ten Have M, Verheul R, Kaasenbrood A, Van Dorsselaer S, Tuithof M, Kleinjan M, et al. Prevalence rates of borderline personality disorder symptoms: a study based on the Netherlands Mental Health Survey and Incidence Study-2. BMC Psychiatry 2016;16:249. [DOI: 10.1186/s12888-016-0939-x] [PMCID: PMC4949762] [PMID: ] - DOI - PMC - PubMed
Thomas 2021
-
- Thomas J, Askie LM, Berlin JA, Elliott JH, Ghersi D, Simmonds M, Takwoingi Y, Tierney JF, Higgins HPT. Prospective approaches to accumulating evidence.. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane 2021. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Vol. Chapter 22. Wiley. Awailable from: www.training.cochrane.org/handbook, Updated February 2021.
Thompson 2019
-
- Thompson KN, Cavelti M, Chanen AM. Psychotic symptoms in adolescents with borderline personality disorder features. Eur Child Adolesc Psychiatry 2019;28(7):985-92. [PMID: ] - PubMed
Thorlund 2009
Tomko 2014
-
- Tomko RL, Trull TJ, Wood PK, Sher KJ. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of Personality Disorders 2014;28(5):734-50. [DOI: 10.1521/pedi_2012_26_093] [PMCID: PMC3864176] [PMID: ] - DOI - PMC - PubMed
Torgersen 2001
Torgersen 2012
-
- Torgersen S. Epidemiology. In: Widiger TA, editors(s). The Oxford Handbook of Personality Disorders. New York (NY): Oxford University Press, 2012:186-205. [DOI: 10.1093/oxfordhb/9780199735013.013.0009] [ISBN: 978-0-19-973501-3] - DOI
Trull 2010
-
- Trull TJ, Jahng S, Tomko RL, Wood PK, Sher KJ. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. Journal of Personality Disorders 2010;24(4):412-26. [DOI: 10.1521/pedi.2010.24.4.412] [PMCID: PMC3771514] [PMID: ] - DOI - PMC - PubMed
Tyrer 2005
-
- Tyrer P, Nur U, Crawford M, Karlsen S, McLean C, Rao B, et al. The Social Functioning Questionnaire: a rapid and robust measure of perceived functioning. International Journal of Social Psychiatry 2005;51(3):265-75. [PMID: ] - PubMed
Tyrer 2015
Van Asselt 2007
Vita 2011
-
- Vita A, De Peri L, Sacchetti E. Antipsychotics, antidepressants, anticonvulsants, and placebo on the symptom dimensions of borderline personality disorder: a meta-analysis of randomized controlled and open-label trials. Journal of Clinical Psychopharmacology 2011;31(5):613-24. [DOI: 10.1097/JCP.0b013e31822c1636] [PMID: ] - DOI - PubMed
Wetterslev 2008
Wetterslev 2009
WHO 1993
-
- World Health Organisation. The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. Geneva (CH): World Health Organization, 1993.
WHO 2021
-
- World Health Organisation. ICD-11 for Mortality and Morbidity Statistics (Version: 05/2021). icd.who.int/browse11/l-m/en (accessed 6 June 2021).
WHO Collaborating Center for Drug Statistics.
-
- WHO Collaborating Center for Drug Statistics. ATC/DDD Index 2021. https://www.whocc.no/atc_ddd_index/ (accessed 03 December 2021).
Widiger 1993
-
- Widiger TA, Trull TJ. Borderline and Narcissistic Personality Disorders. In: PB Sutker, HE Adams, editors(s). Comprehensive Handbook of Psychopathology. New York, NY: Plenum, 1993:371-94.
Widiger 1998
-
- Widiger TA. Invited essay: Sex biasesin the diagnosis of personality disorders. Journal of Personality Disorders 1998;12(2):95-118. - PubMed
Wood 2008
-
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336(7644):601-5. [DOI: 10.1136/bmj.39465.451748.AD] [PMCID: PMC2267990] [PMID: ] - DOI - PMC - PubMed
Yen 2021
-
- Yen S, Peters JR, Nishar S, Grilo CM, Sanislow CA, Shea MT, et al. Association of borderline personality disorder criteria with suicide attempts: findings from the Collaborative Longitudinal Study of Personality Disorders over 10 years of follow-up. JAMA Psychiatry 2021;78(2):187-94. [DOI: 10.1001/jamapsychiatry.2020.3598] [PMCID: PMC7675214] [PMID: ] - DOI - PMC - PubMed
Zanarini 1998
Zanarini 2004a
Zanarini 2004b
Zanarini 2005
Zanarini 2010
-
- Zanarini MC, Frankenburg FR, Reich DB, Fitzmaurice G. The 10-year course of psychosocial functioning among patients with borderline personality disorder and axis II comparison subjects. Acta Psychiatrica Scandinavica 2010;122(2):103-9. [DOI: 10.1111/j.1600-0447.2010.01543.x] [PMCID: PMC3876887] [PMID: ] - DOI - PMC - PubMed
Zanarini 2012
-
- Zanarini MC, Frankenburg FR, Reich B, Fitzmaurice G. Attainment and stability of sustained symptomatic remission and recovery among patients with borderline personality disorder and axis II comparison subjects: a 16-year prospective follow-up study. American Journal of Psychiatry 2012;169(5):476-83. [DOI: 10.1176/appi.ajp.2011.11101550] [PMCID: PMC3509999] [PMID: ] - DOI - PMC - PubMed
Zanarini 2015
-
- Zanarini MC, Frankenburg FR, Reich DB, Harned AL, Fitzmaurice GM. Rates of psychotropic medication use reported by borderline patients and axis II comparison subjects over 16 years of prospective follow-up. Journal of Clinical Psychopharmacology 2015;35(1):63-7. [DOI: 10.1097/JCP.0000000000000232] [PMCID: PMC4276426] [PMID: ] - DOI - PMC - PubMed
References to other published versions of this review
Binks 2006
Stoffers 2010
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

