Fulminant lung fibrosis in non-resolvable COVID-19 requiring transplantation
- PMID: 36375315
- PMCID: PMC9667270
- DOI: 10.1016/j.ebiom.2022.104351
Fulminant lung fibrosis in non-resolvable COVID-19 requiring transplantation
Abstract
Background: Coronavirus Disease 2019 (COVID-19) can lead to the development of acute respiratory distress syndrome (ARDS). In some patients with non-resolvable (NR) COVID-19, lung injury can progress rapidly to the point that lung transplantation is the only viable option for survival. This fatal progression of lung injury involves a rapid fibroproliferative response and takes on average 15 weeks from initial symptom presentation. Little is known about the mechanisms that lead to this fulminant lung fibrosis (FLF) in NR-COVID-19.
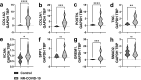
Methods: Using a pre-designed unbiased PCR array for fibrotic markers, we analyzed the fibrotic signature in a subset of NR-COVID-19 lungs. We compared the expression profile against control lungs (donor lungs discarded for transplantation), and explanted tissue from patients with idiopathic pulmonary fibrosis (IPF). Subsequently, RT-qPCR, Western blots and immunohistochemistry were conducted to validate and localize selected pro-fibrotic targets. A total of 23 NR-COVID-19 lungs were used for RT-qPCR validation.
Findings: We revealed a unique fibrotic gene signature in NR-COVID-19 that is dominated by a hyper-expression of pro-fibrotic genes, including collagens and periostin. Our results also show a significantly increased expression of Collagen Triple Helix Repeat Containing 1(CTHRC1) which co-localized in areas rich in alpha smooth muscle expression, denoting myofibroblasts. We also show a significant increase in cytokeratin (KRT) 5 and 8 expressing cells adjacent to fibroblastic areas and in areas of apparent epithelial bronchiolization.
Interpretation: Our studies may provide insights into potential cellular mechanisms that lead to a fulminant presentation of lung fibrosis in NR-COVID-19.
Funding: National Institute of Health (NIH) Grants R01HL154720, R01DK122796, R01DK109574, R01HL133900, and Department of Defense (DoD) Grant W81XWH2110032 to H.K.E. NIH Grants: R01HL138510 and R01HL157100, DoD Grant W81XWH-19-1-0007, and American Heart Association Grant: 18IPA34170220 to H.K.-Q. American Heart Association: 19CDA34660279, American Lung Association: CA-622265, Parker B. Francis Fellowship, 1UL1TR003167-01 and The Center for Clinical and Translational Sciences, McGovern Medical School to X.Y.
Keywords: CTHRC1; ECMO; Extracellular matrix; Extracorporeal life support; KRT5; KRT8; Periostin (POSTN); Post-acute SARS-CoV-2 sequelae (PASC).
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare no conflict of interest except for HJH who served as speaker and advisory panel member for Boehringer Ingelheim for Nintedanib.
Figures






References
-
- CDC . 2020. COVID-19 pandemic planning scenarios.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

