The effect of rebamipide on non-steroidal anti-inflammatory drug-induced gastro-enteropathy: a multi-center, randomized pilot study
- PMID: 36375487
- PMCID: PMC9666262
- DOI: 10.3904/kjim.2021.216
The effect of rebamipide on non-steroidal anti-inflammatory drug-induced gastro-enteropathy: a multi-center, randomized pilot study
Abstract
Background/aims: Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly-used medications, and ailments such as arthritis or heart disease, require long-term use of these drugs, which can induce gastroenteropathy with bleeding and ulcers. This study investigated the associations between efficacy, safety, and gastrointestinal symptoms linked to rebamipide and proton pump inhibitor administration in patients requiring long-term NSAID use.
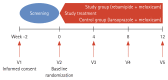
Methods: This study was a multi-center, randomized, open-labeled, pilot design.
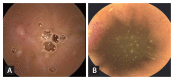
Results: Thirty-three patients were included. Of these, 15 were included in the study group and 18 were in the control group. NSAID-induced gastric ulcers, which were the primary outcome of this study, did not occur in either the study or control group. Changes in the number of small bowel erosions and ulcers were -0.6 ± 3.06 in the study group and 1.33 ± 4.71 in the control group. The number of subjects with mucosal breaks (defined as multiple erosions and/or ulcers) was three (20%) in the study group and six (40%) in the control group (p = 0.427). No serious adverse events occurred in either group. However, dyspepsia and skin rashes occurred in six patients (31.58%) in the study group and 13 (65%) in the control group (p = 0.036).
Conclusion: Although statistically significant differences were not generated, possibly as a result of the small sample size, mucosal breaks observed via capsule endoscopy revealed that rebamipide was likely to be more effective than lansoprazole in preventing small intestine damage caused by NSAIDs. Furthermore, fewer side-effects emerged with rebamipide.
Keywords: Anti-inflammatory agents, non-steroidal; Gastrointestinal diseases; Proton pump inhibitors; Rebamipide; Small bowel injury.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Laine L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology. 2001;120:594–606. - PubMed
-
- Maiden L. Capsule endoscopic diagnosis of nonsteroidal antiinflammatory drug-induced enteropathy. J Gastroenterol. 2009;44(Suppl 19):64–71. - PubMed
-
- Fujimori S, Takahashi Y, Gudis K, et al. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy. J Gastroenterol. 2011;46:57–64. - PubMed
-
- Regula J, Butruk E, Dekkers CP, et al. Prevention of NSAID-associated gastrointestinal lesions: a comparison study pantoprazole versus omeprazole. Am J Gastroenterol. 2006;101:1747–1755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources