P2X7 Receptor and Purinergic Signaling: Orchestrating Mitochondrial Dysfunction in Neurodegenerative Diseases
- PMID: 36376084
- PMCID: PMC9665882
- DOI: 10.1523/ENEURO.0092-22.2022
P2X7 Receptor and Purinergic Signaling: Orchestrating Mitochondrial Dysfunction in Neurodegenerative Diseases
Abstract
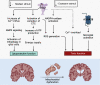
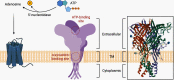
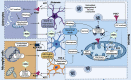
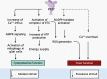
Mitochondrial dysfunction is one of the basic hallmarks of cellular pathology in neurodegenerative diseases. Since the metabolic activity of neurons is highly dependent on energy supply, nerve cells are especially vulnerable to impaired mitochondrial function. Besides providing oxidative phosphorylation, mitochondria are also involved in controlling levels of second messengers such as Ca2+ ions and reactive oxygen species (ROS). Interestingly, the critical role of mitochondria as producers of ROS is closely related to P2XR purinergic receptors, the activity of which is modulated by free radicals. Here, we review the relationships between the purinergic signaling system and affected mitochondrial function. Purinergic signaling regulates numerous vital biological processes in the CNS. The two main purines, ATP and adenosine, act as excitatory and inhibitory neurotransmitters, respectively. Current evidence suggests that purinergic signaling best explains how neuronal activity is related to neuronal electrical activity and energy homeostasis, especially in the development of Alzheimer's and Parkinson's diseases. In this review, we focus on the mechanisms underlying the involvement of the P2RX7 purinoreceptor in triggering mitochondrial dysfunction during the development of neurodegenerative disorders. We also summarize various avenues by which the purine signaling pathway may trigger metabolic dysfunction contributing to neuronal death and the inflammatory activation of glial cells. Finally, we discuss the potential role of the purinergic system in the search for new therapeutic approaches to treat neurodegenerative diseases.
Keywords: exosomes; mitochondrion; oxidative stress; purinergic metabolome; tau protein; α-synuclein.
Copyright © 2022 Zelentsova et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Agosta F, Dalla Libera D, Spinelli EG, Finardi A, Canu E, Bergami A, Bocchio Chiavetto L, Baronio M, Comi G, Martino G, Matteoli M, Magnani G, Verderio C, Furlan R (2014) Myeloid microvesicles in cerebrospinal fluid are associated with myelin damage and neuronal loss in mild cognitive impairment and Alzheimer disease. Ann Neurol 76:813–825. 10.1002/ana.24235 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
