Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum
- PMID: 36376214
- PMCID: PMC10318934
- DOI: 10.1016/j.vaccine.2022.11.002
Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum
Abstract
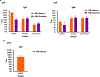
Syphilis continues to be a significant public health concern worldwide. The disease is endemic in many low- and middle-income countries, and rates have risen sharply in high-income countries over the last decade. The continued prevalence of infectious and congenital syphilis worldwide highlights the need for the development of an effective syphilis vaccine to complement public health measures for syphilis control. The complex, multi-stage course of syphilis infection necessitates a holistic approach to the development of an effective vaccine, in which immunization prevents both the localized stage of infection (typified by the highly infectious chancre) and the disseminated stages of infection (typified by the secondary rash, neurosyphilis, and destructive tertiary lesions, as well as congenital syphilis). Inhibiting development of the infectious chancre would reduce transmission thus providing community- level protection, while preventing dissemination would provide individual-level protection by reducing serious sequelae and may also provide community level protection by reducing shedding during secondary syphilis. In the current study we build upon prior investigations which demonstrated that immunizations with individual, well characterized T. pallidum TprK, TprC, and Tp0751 peptides elicits partial protection against infection in the animal model. Specifically, we show here that immunization with a TprC/TprK/Tp0751 tri-antigen cocktail protects animals from progressive syphilis lesions and substantially inhibits dissemination of the infection.
Keywords: Bacterium; Syphilis; Treponema pallidum; Vaccine.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Caroline Cameron has patent issued to University of Victoria.
Figures






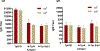


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

