Low intensity near-infrared light promotes bone regeneration via circadian clock protein cryptochrome 1
- PMID: 36376275
- PMCID: PMC9663728
- DOI: 10.1038/s41368-022-00207-y
Low intensity near-infrared light promotes bone regeneration via circadian clock protein cryptochrome 1
Abstract
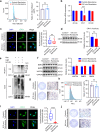
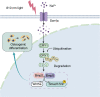
Bone regeneration remains a great clinical challenge. Low intensity near-infrared (NIR) light showed strong potential to promote tissue regeneration, offering a promising strategy for bone defect regeneration. However, the effect and underlying mechanism of NIR on bone regeneration remain unclear. We demonstrated that bone regeneration in the rat skull defect model was significantly accelerated with low-intensity NIR stimulation. In vitro studies showed that NIR stimulation could promote the osteoblast differentiation in bone mesenchymal stem cells (BMSCs) and MC3T3-E1 cells, which was associated with increased ubiquitination of the core circadian clock protein Cryptochrome 1 (CRY1) in the nucleus. We found that the reduction of CRY1 induced by NIR light activated the bone morphogenetic protein (BMP) signaling pathways, promoting SMAD1/5/9 phosphorylation and increasing the expression levels of Runx2 and Osterix. NIR light treatment may act through sodium voltage-gated channel Scn4a, which may be a potential responder of NIR light to accelerate bone regeneration. Together, these findings suggest that low-intensity NIR light may promote in situ bone regeneration in a CRY1-dependent manner, providing a novel, efficient and non-invasive strategy to promote bone regeneration for clinical bone defects.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cryptochrome 1 promotes osteogenic differentiation of human osteoblastic cells via Wnt/β-Catenin signaling.Life Sci. 2018 Nov 1;212:129-137. doi: 10.1016/j.lfs.2018.09.053. Epub 2018 Oct 2. Life Sci. 2018. PMID: 30290183
-
Sulfuretin promotes osteoblastic differentiation in primary cultured osteoblasts and in vivo bone healing.Oncotarget. 2016 Nov 29;7(48):78320-78330. doi: 10.18632/oncotarget.12460. Oncotarget. 2016. PMID: 27713171 Free PMC article.
-
Harmine promotes osteoblast differentiation through bone morphogenetic protein signaling.Biochem Biophys Res Commun. 2011 Jun 3;409(2):260-5. doi: 10.1016/j.bbrc.2011.05.001. Epub 2011 May 6. Biochem Biophys Res Commun. 2011. PMID: 21570953
-
Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase A signaling.Int J Mol Med. 2016 Apr;37(4):901-10. doi: 10.3892/ijmm.2016.2490. Epub 2016 Feb 18. Int J Mol Med. 2016. PMID: 26935542 Free PMC article.
-
Pilose antler aqueous extract promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells by stimulating the BMP-2/Smad1, 5/Runx2 signaling pathway.Chin J Nat Med. 2019 Oct;17(10):756-767. doi: 10.1016/S1875-5364(19)30092-5. Chin J Nat Med. 2019. PMID: 31703756
Cited by
-
Hydrogel microspheres for bone regeneration through regulation of the regenerative microenvironment.Biomater Transl. 2024 Sep 28;5(3):205-235. doi: 10.12336/biomatertransl.2024.03.002. eCollection 2024. Biomater Transl. 2024. PMID: 39734698 Free PMC article. Review.
-
Osteoporotic osseointegration: therapeutic hallmarks and engineering strategies.Theranostics. 2024 Jun 17;14(10):3859-3899. doi: 10.7150/thno.96516. eCollection 2024. Theranostics. 2024. PMID: 38994021 Free PMC article. Review.
-
The Effects and Mechanisms of PBM Therapy in Accelerating Orthodontic Tooth Movement.Biomolecules. 2023 Jul 17;13(7):1140. doi: 10.3390/biom13071140. Biomolecules. 2023. PMID: 37509176 Free PMC article. Review.
-
Enhancing Biodegradable Bone Plate Performance: Stereocomplex Polylactic Acid for Improved Mechanical Properties and Near-Infrared Transparency.Biomacromolecules. 2025 Apr 14;26(4):2390-2401. doi: 10.1021/acs.biomac.4c01768. Epub 2025 Mar 25. Biomacromolecules. 2025. PMID: 40130648 Free PMC article.
-
The roles of Cryptochrome-1: the circadian clock as a control point in cancer therapy.J Transl Med. 2025 Jun 17;23(1):669. doi: 10.1186/s12967-025-06702-0. J Transl Med. 2025. PMID: 40528171 Free PMC article. Review.
References
-
- De Santis, R., Guarino, V. & Ambrosio, L. In Bone Repair Biomaterials (eds. Planell, J.A.et al.) 252–270 (Woodhead Publishing, 2009).
-
- Sun H, et al. CD271 antibody-functionalized microspheres capable of selective recruitment of reparative endogenous stem cells for in situ bone regeneration. Biomaterials. 2022;280:121243. - PubMed
-
- Holt, B. D., Wright, Z. M., Arnold, A. M. & Sydlik, S. A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol.10.1002/wnan.1437 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

