A flexible adhesive surface electrode array capable of cervical electroneurography during a sequential autonomic stress challenge
- PMID: 36376365
- PMCID: PMC9663551
- DOI: 10.1038/s41598-022-21817-w
A flexible adhesive surface electrode array capable of cervical electroneurography during a sequential autonomic stress challenge
Abstract
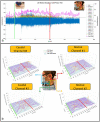
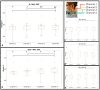
This study introduces a flexible, adhesive-integrated electrode array that was developed to enable non-invasive monitoring of cervical nerve activity. The device uses silver-silver chloride as the electrode material of choice and combines it with an electrode array consisting of a customized biopotential data acquisition unit and integrated graphical user interface (GUI) for visualization of real-time monitoring. Preliminary testing demonstrated this electrode design can achieve a high signal to noise ratio during cervical neural recordings. To demonstrate the capability of the surface electrodes to detect changes in cervical neuronal activity, the cold-pressor test (CPT) and a timed respiratory challenge were employed as stressors to the autonomic nervous system. This sensor system recording, a new technique, was termed Cervical Electroneurography (CEN). By applying a custom spike sorting algorithm to the electrode measurements, neural activity was classified in two ways: (1) pre-to-post CPT, and (2) during a timed respiratory challenge. Unique to this work: (1) rostral to caudal channel position-specific (cephalad to caudal) firing patterns and (2) cross challenge biotype-specific change in average CEN firing, were observed with both CPT and the timed respiratory challenge. Future work is planned to develop an ambulatory CEN recording device that could provide immediate notification of autonomic nervous system activity changes that might indicate autonomic dysregulation in healthy subjects and clinical disease states.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Jänig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992;15:475–481. - PubMed
-
- Levy MN, Koeppen BM, Stanton BA. Berne & Levy Principles of Physiology E-Book. Elsevier Health Sciences; 2005.

