When cell death goes wrong: inflammatory outcomes of failed apoptosis and mitotic cell death
- PMID: 36376381
- PMCID: PMC9661468
- DOI: 10.1038/s41418-022-01082-0
When cell death goes wrong: inflammatory outcomes of failed apoptosis and mitotic cell death
Abstract
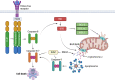
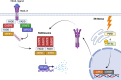
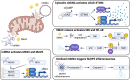
Apoptosis is a regulated cellular pathway that ensures that a cell dies in a structured fashion to prevent negative consequences for the tissue or the organism. Dysfunctional apoptosis is a hallmark of numerous pathologies, and treatments for various diseases are successful based on the induction of apoptosis. Under homeostatic conditions, apoptosis is a non-inflammatory event, as the activation of caspases ensures that inflammatory pathways are disabled. However, there is an increasing understanding that under specific conditions, such as caspase inhibition, apoptosis and the apoptotic machinery can be re-wired into a process which is inflammatory. In this review we discuss how the death receptor and mitochondrial pathways of apoptosis can activate inflammation. Furthermore, we will highlight how cell death due to mitotic stress might be a special case when it comes to cell death and the induction of inflammation.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

