SNORD88C guided 2'-O-methylation of 28S rRNA regulates SCD1 translation to inhibit autophagy and promote growth and metastasis in non-small cell lung cancer
- PMID: 36376383
- PMCID: PMC9950066
- DOI: 10.1038/s41418-022-01087-9
SNORD88C guided 2'-O-methylation of 28S rRNA regulates SCD1 translation to inhibit autophagy and promote growth and metastasis in non-small cell lung cancer
Abstract
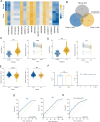
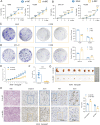
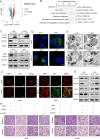
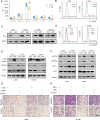
Small nucleolar RNAs (snoRNAs) have been shown to play critical regulatory roles in cancer development. SNORD88C, which located at the intronic region of C19orf48 in chromosome 19q.33 with a 97-nt length was screened through database and snoRNA-sequencing. We firstly verified this snoRNA was up-regulated in tissue and plasma and served as a non-invasive diagnostic biomarker; then confirmed that SNORD88C promoted proliferation and metastasis of NSCLC in vitro and in vivo. Mechanistically, SNORD88C promoted 2'-O-methylation modification at the C3680 site on 28S rRNA and in turn enhanced downstream SCD1 translation, a central lipogenic enzyme for the synthesis of MUFA that can inhibit autophagy by regulating lipid peroxidation and mTOR, providing the novel insight into the regulation of SNORD88C in NSCLC.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

