The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source
- PMID: 36376388
- PMCID: PMC9836935
- DOI: 10.1038/s41557-022-01067-z
The gas-phase formation mechanism of iodic acid as an atmospheric aerosol source
Abstract
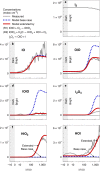
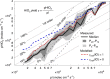
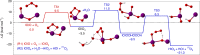
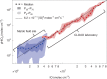
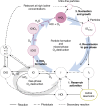
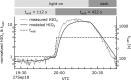
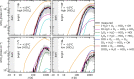
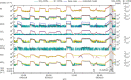
Iodine is a reactive trace element in atmospheric chemistry that destroys ozone and nucleates particles. Iodine emissions have tripled since 1950 and are projected to keep increasing with rising O3 surface concentrations. Although iodic acid (HIO3) is widespread and forms particles more efficiently than sulfuric acid, its gas-phase formation mechanism remains unresolved. Here, in CLOUD atmospheric simulation chamber experiments that generate iodine radicals at atmospherically relevant rates, we show that iodooxy hypoiodite, IOIO, is efficiently converted into HIO3 via reactions (R1) IOIO + O3 → IOIO4 and (R2) IOIO4 + H2O → HIO3 + HOI + (1)O2. The laboratory-derived reaction rate coefficients are corroborated by theory and shown to explain field observations of daytime HIO3 in the remote lower free troposphere. The mechanism provides a missing link between iodine sources and particle formation. Because particulate iodate is readily reduced, recycling iodine back into the gas phase, our results suggest a catalytic role of iodine in aerosol formation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Kreidenweis SM, Seinfeld JH. Nucleation of sulfuric acid–water and methanesulfonic acid–water solution particles: implications for the atmospheric chemistry of organosulfur species. Atmos. Environ. 1988;22:283–296. doi: 10.1016/0004-6981(88)90034-0. - DOI
-
- Kuang, C., McMurry, P. H., McCormick, A. V. & Eisele, F. L. Dependence of nucleation rates on sulfuric acid vapor concentration in diverse atmospheric locations. J. Geophys. Res. Atmos. 10.1029/2007JD009253 (2008).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

