The solvent chosen for the manufacturing of electrospun polycaprolactone scaffolds influences cell behavior of lung cancer cells
- PMID: 36376404
- PMCID: PMC9663546
- DOI: 10.1038/s41598-022-23655-2
The solvent chosen for the manufacturing of electrospun polycaprolactone scaffolds influences cell behavior of lung cancer cells
Abstract
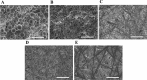
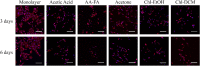
The development of a trustworthy in vitro lung cancer model is essential to better understand the illness, find novel biomarkers, and establish new treatments. Polycaprolactone (PCL) electrospun nanofibers are a cost-effective and ECM-like approach for 3D cell culture. However, the solvent used to prepare the polymer solution has a significant impact on the fiber morphology and, consequently, on the cell behavior. Hence, the present study evaluated the effect of the solvent employed in the manufacturing on the physical properties of 15%-PCL electrospun scaffolds and consequently, on cell behavior of NCI-H1975 lung adenocarcinoma cells. Five solvents mixtures (acetic acid, acetic acid-formic acid (3:1, v/v), acetone, chloroform-ethanol (7:3, v/v), and chloroform-dichloromethane (7:3, v/v)) were tested. The highest cell viability ([Formula: see text] = 33.4%) was found for cells cultured on chloroform-ethanol (7:3) PCL scaffolds. Chloroform-dichloromethane (7:3) PCL scaffolds exhibited a roughness that enhanced the quality of electrospun filament, in terms of cell viability. Our findings highlighted the influence of the solvent on fiber morphology and protein adsorption capacity of nanofilaments. Consequently, these features directly affected cell attachment, morphology, and viability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Investigation of the batch-to-batch inconsistencies of Collagen in PCL-Collagen nanofibers.Mater Sci Eng C Mater Biol Appl. 2019 Feb 1;95:217-225. doi: 10.1016/j.msec.2018.10.057. Epub 2018 Oct 26. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 30573244
-
Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation.Biomaterials. 2017 Jan;115:115-127. doi: 10.1016/j.biomaterials.2016.11.018. Epub 2016 Nov 15. Biomaterials. 2017. PMID: 27886552 Free PMC article.
-
Preparation of cell culture scaffolds using polycaprolactone/quince seed mucilage.Int J Biol Macromol. 2020 Jul 15;155:1270-1276. doi: 10.1016/j.ijbiomac.2019.11.096. Epub 2019 Nov 11. Int J Biol Macromol. 2020. PMID: 31726121
-
Electrospun polycaprolactone/hydroxyapatite/ZnO nanofibers as potential biomaterials for bone tissue regeneration.J Mater Sci Mater Med. 2019 Apr 22;30(5):51. doi: 10.1007/s10856-019-6255-5. J Mater Sci Mater Med. 2019. PMID: 31011810
-
In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering.Mater Sci Eng C Mater Biol Appl. 2020 Sep;114:111056. doi: 10.1016/j.msec.2020.111056. Epub 2020 May 6. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32994008
Cited by
-
Exosome-loaded biomaterials for tendon/ligament repair.Biomater Transl. 2024 Jun 28;5(2):129-143. doi: 10.12336/biomatertransl.2024.02.004. eCollection 2024. Biomater Transl. 2024. PMID: 39351162 Free PMC article. Review.
-
Screening of electrospun PS/PCL scaffolds for three-dimensional triple negative breast cancer cell culture: impact of solvent, hydrophobicity, and setup orientation.Sci Rep. 2025 Jul 1;15(1):22110. doi: 10.1038/s41598-025-05871-8. Sci Rep. 2025. PMID: 40594674 Free PMC article.
References
-
- Forsythe ML, Alwithenani A, Bethune D, Castonguay M, Drucker A, Flowerdew G, French D, Fris J, Greer W, Henteleff H, MacNeil M, Marignani P, Morzycki W, Plourde M, Snow S, Xu Z. Molecular profiling of non-small cell lung cancer. PLoS One. 2020;15:e0236580. doi: 10.1371/journal.pone.0236580. - DOI - PMC - PubMed
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beth Beasley M, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Sound Tsao M, Wistuba I. On behalf of the WHO panel, the 2015 World Health Organization classification of lung tumors. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

