Mathematical analysis of phototransduction reaction parameters in rods and cones
- PMID: 36376413
- PMCID: PMC9663442
- DOI: 10.1038/s41598-022-23069-0
Mathematical analysis of phototransduction reaction parameters in rods and cones
Abstract
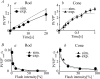
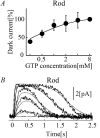
Retinal photoreceptor cells, rods and cones, convert photons of light into chemical and electrical signals as the first step of the visual transduction cascade. Although the chemical processes in the phototransduction system are very similar to each other in these photoreceptors, the light sensitivity and time resolution of the photoresponse in rods are functionally different than those in the photoresponses of cones. To systematically investigate how photoresponses are divergently regulated in rods and cones, we have developed a detailed mathematical model on the basis of the Hamer model. The current model successfully reconstructed light intensity-, ATP- and GTP-dependent changes in concentrations of phosphorylated visual pigments (VPs), activated transducins (Tr*s) and phosphodiesterases (PDEs) in rods and cones. In comparison to rods, the lower light sensitivity of cones was attributed not only to the lower affinity of activated VPs for Trs but also to the faster desensitization of the VPs. The assumption of an intermediate inactive state, MIIi, in the thermal decay of activated VPs was essential for inducing faster inactivation of VPs in rods, and possibly also in cones.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Low activation and fast inactivation of transducin in carp cones.J Biol Chem. 2012 Nov 30;287(49):41186-94. doi: 10.1074/jbc.M112.403717. Epub 2012 Oct 8. J Biol Chem. 2012. PMID: 23045532 Free PMC article.
-
Activation and quenching of the phototransduction cascade in retinal cones as inferred from electrophysiology and mathematical modeling.Mol Vis. 2015 Mar 7;21:244-63. eCollection 2015. Mol Vis. 2015. PMID: 25866462 Free PMC article.
-
[Physiology of the visual retinal signal: From phototransduction to the visual cycle].J Fr Ophtalmol. 2017 Mar;40(3):239-250. doi: 10.1016/j.jfo.2016.12.006. Epub 2017 Mar 17. J Fr Ophtalmol. 2017. PMID: 28318721 Review. French.
-
Mechanistic basis for the failure of cone transducin to translocate: why cones are never blinded by light.J Neurosci. 2010 May 19;30(20):6815-24. doi: 10.1523/JNEUROSCI.0613-10.2010. J Neurosci. 2010. PMID: 20484624 Free PMC article.
Cited by
-
Dynamic Chromatin Accessibility and Transcriptional Regulation in the Eyes of Red Tilapia (Oreochromis sp.) in Response to Wintering Stress.Mar Biotechnol (NY). 2025 Feb 12;27(1):47. doi: 10.1007/s10126-025-10424-1. Mar Biotechnol (NY). 2025. PMID: 39937323
-
Lycium barbarum L.: a potential botanical drug for preventing and treating retinal cell apoptosis.Front Pharmacol. 2025 Mar 20;16:1571554. doi: 10.3389/fphar.2025.1571554. eCollection 2025. Front Pharmacol. 2025. PMID: 40183099 Free PMC article. Review.
-
Light wavelength modulates search behavior performance in zebrafish.Sci Rep. 2024 Jul 17;14(1):16533. doi: 10.1038/s41598-024-67262-9. Sci Rep. 2024. PMID: 39019915 Free PMC article.
References
-
- Kawamura S, Tachibanaki S. Explaining the functional differences of rods versus cones. WIREs Membr. Transp. Signal. 2012;1:675–683.
-
- Kawamura S, Tachibanaki S. Phototransduction in rods and cones. In: Furukawa T, Hurley J, Kawamura S, editors. Vertebrate Photoreceptors. Springer; 2014. pp. 23–45.
-
- Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. - PubMed
-
- Baylor DA. Photoreceptor signals and vision, proctor lecture. Investig. Ophthalmol. Vis. Sci. 1987;28:34–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

