The protein conformational basis of isoflavone biosynthesis
- PMID: 36376429
- PMCID: PMC9663428
- DOI: 10.1038/s42003-022-04222-x
The protein conformational basis of isoflavone biosynthesis
Abstract
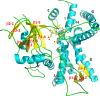
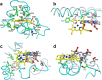
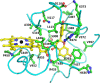
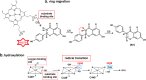
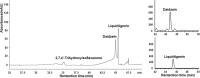
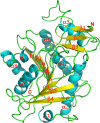
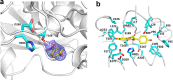
Isoflavonoids play important roles in plant defense and also exhibit a range of mammalian health-promoting activities. Their biosynthesis is initiated by two enzymes with unusual catalytic activities; 2-hydroxyisoflavanone synthase (2-HIS), a membrane-bound cytochrome P450 catalyzing a coupled aryl-ring migration and hydroxylation, and 2-hydroxyisoflavanone dehydratase (2-HID), a member of a large carboxylesterase family that paradoxically catalyzes dehydration of 2-hydroxyisoflavanones to isoflavone. Here we report the crystal structures of 2-HIS from Medicago truncatula and 2-HID from Pueraria lobata. The 2-HIS structure reveals a unique cytochrome P450 conformation and heme and substrate binding mode that facilitate the coupled aryl-ring migration and hydroxylation reactions. The 2-HID structure reveals the active site architecture and putative catalytic residues for the dual dehydratase and carboxylesterase activities. Mutagenesis studies revealed key residues involved in substrate binding and specificity. Understanding the structural basis of isoflavone biosynthesis will facilitate the engineering of new bioactive isoflavonoids.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
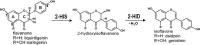
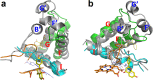
References
-
- Dixon, R. A. Isoflavonoids: biochemistry, molecular biology and biological functions. in Comprehensive Natural Products Chemistry (ed. Sankawa, U.) 773–823 (Elsevier, 1999).
-
- Dixon RA, Ferreira D. Molecules of interest: genistein. Phytochemistry. 2002;60:205–211. - PubMed
-
- Palevitz BA. Soybeans hit main street. Scientist. 2000;14:8–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

