Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake
- PMID: 36376430
- PMCID: PMC9663570
- DOI: 10.1038/s41598-022-20772-w
Zinc- and magnesium-doped hydroxyapatite-urea nanohybrids enhance wheat growth and nitrogen uptake
Abstract
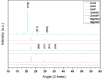
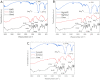
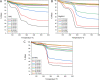
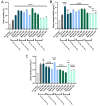
The ongoing and unrestrained application of nitrogen fertilizer to agricultural lands has been directly linked to climate change and reductions in biodiversity. The agricultural sector needs a technological upgrade to adopt sustainable methods for maintaining high yield. We report synthesis of zinc and magnesium doped and undoped hydroxyapatite nanoparticles, and their urea nanohybrids, to sustainably deliver nitrogen to wheat. The urea nanohybrids loaded with up to 42% nitrogen were used as a new source of nitrogen and compared with a conventional urea-based fertilizer for efficient and sufficient nitrogen delivery to pot-grown wheat. Doping with zinc and magnesium manipulated the hydroxyapatite crystallinity for smaller size and higher nitrogen loading capacity. Interestingly, 50% and 25% doses of urea nanohybrids significantly boosted the wheat growth and yield compared with 100% doses of urea fertilizer. In addition, the nutritional elements uptake and grain protein and phospholipid levels were significantly enhanced in wheat treated with nanohybrids. These results demonstrate the potential of the multi-nutrient complexes, the zinc and magnesium doped and undoped hydroxyapatite-urea nanoparticles, as nitrogen delivery agents that reduce nitrogen inputs by at least 50% while maintaining wheat plant growth and nitrogen uptake to the same level as full-dose urea treatments.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Metal doped nitrogenous hydroxyapatite nanohybrids slowly release nitrogen to crops and mitigate ammonia volatilization: An impact assessment.NanoImpact. 2022 Oct;28:100424. doi: 10.1016/j.impact.2022.100424. Epub 2022 Sep 7. NanoImpact. 2022. PMID: 36087836
-
Slow-release nitrogen fertilizers enhance growth, yield, NUE in wheat crop and reduce nitrogen losses under an arid environment.Environ Sci Pollut Res Int. 2021 Aug;28(32):43528-43543. doi: 10.1007/s11356-021-13700-4. Epub 2021 Apr 9. Environ Sci Pollut Res Int. 2021. PMID: 33834341 Free PMC article.
-
Controlled nitrogen transformation in chemo-amended urea improves nitrogen use efficiency and productivity of wheat grown on alkaline calcareous soil.Environ Sci Pollut Res Int. 2022 Apr;29(19):28700-28713. doi: 10.1007/s11356-021-17837-0. Epub 2022 Jan 6. Environ Sci Pollut Res Int. 2022. PMID: 34988797
-
Bacterial impregnation of mineral fertilizers improves yield and nutrient use efficiency of wheat.J Sci Food Agric. 2017 Aug;97(11):3685-3690. doi: 10.1002/jsfa.8228. Epub 2017 Feb 23. J Sci Food Agric. 2017. PMID: 28106248
-
Facile coating of micronutrient zinc for slow release urea and its agronomic effects on field grown wheat (Triticum aestivum L.).Sci Total Environ. 2022 Sep 10;838(Pt 1):155965. doi: 10.1016/j.scitotenv.2022.155965. Epub 2022 May 16. Sci Total Environ. 2022. PMID: 35588805
Cited by
-
Application of nano-urea in conventional flood-irrigated Boro rice in Bangladesh and nitrogen losses investigation.Heliyon. 2024 Aug 30;10(17):e37150. doi: 10.1016/j.heliyon.2024.e37150. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296209 Free PMC article.
-
Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability.Front Microbiol. 2023 May 3;14:1133968. doi: 10.3389/fmicb.2023.1133968. eCollection 2023. Front Microbiol. 2023. PMID: 37206335 Free PMC article. Review.
-
Nanohybrid-enabled smart platforms for biostimulation and immunoengineering of plants.Mater Today Bio. 2025 Jun 14;33:101989. doi: 10.1016/j.mtbio.2025.101989. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40636027 Free PMC article. Review.
References
-
- Savci S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012;3:73.
-
- Berners-Lee M, Kennelly C, Watson R, Hewitt CN. Current global food production is sufficient to meet human nutritional needs in 2050 provided there is radical societal adaptation. Elem. Sci. Anthrop. 2018;6:66. doi: 10.1525/elementa.310. - DOI
-
- Byrnes B. Environmental effects of N fertilizer use—An overview. Fertil. Res. 1990;26:209–215.
-
- Zhu ZL, Chen D. Nitrogen fertilizer use in China-contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002;63:117–127.
-
- Meisinger J, Delgado J. Principles for managing nitrogen leaching. J. Soil Water Conserv. 2002;57:485–498.

