Model simulations unveil the structure-function-dynamics relationship of the cerebellar cortical microcircuit
- PMID: 36376444
- PMCID: PMC9663576
- DOI: 10.1038/s42003-022-04213-y
Model simulations unveil the structure-function-dynamics relationship of the cerebellar cortical microcircuit
Abstract
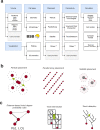
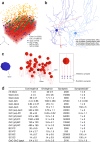
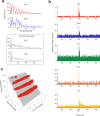
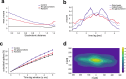
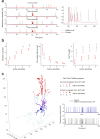
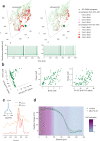
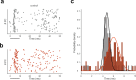
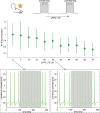
The cerebellar network is renowned for its regular architecture that has inspired foundational computational theories. However, the relationship between circuit structure, function and dynamics remains elusive. To tackle the issue, we developed an advanced computational modeling framework that allows us to reconstruct and simulate the structure and function of the mouse cerebellar cortex using morphologically realistic multi-compartmental neuron models. The cerebellar connectome is generated through appropriate connection rules, unifying a collection of scattered experimental data into a coherent construct and providing a new model-based ground-truth about circuit organization. Naturalistic background and sensory-burst stimulation are used for functional validation against recordings in vivo, monitoring the impact of cellular mechanisms on signal propagation, inhibitory control, and long-term synaptic plasticity. Our simulations show how mossy fibers entrain the local neuronal microcircuit, boosting the formation of columns of activity travelling from the granular to the molecular layer providing a new resource for the investigation of local microcircuit computation and of the neural correlates of behavior.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Model-Driven Analysis of Eyeblink Classical Conditioning Reveals the Underlying Structure of Cerebellar Plasticity and Neuronal Activity.IEEE Trans Neural Netw Learn Syst. 2017 Nov;28(11):2748-2762. doi: 10.1109/TNNLS.2016.2598190. IEEE Trans Neural Netw Learn Syst. 2017. PMID: 27608482
-
Adaptive-filter models of the cerebellum: computational analysis.Cerebellum. 2008;7(4):567-71. doi: 10.1007/s12311-008-0067-3. Cerebellum. 2008. PMID: 18972182
-
Dynamic Redistribution of Plasticity in a Cerebellar Spiking Neural Network Reproducing an Associative Learning Task Perturbed by TMS.Int J Neural Syst. 2018 Nov;28(9):1850020. doi: 10.1142/S012906571850020X. Epub 2018 Apr 24. Int J Neural Syst. 2018. PMID: 29914314
-
The organization of plasticity in the cerebellar cortex: from synapses to control.Prog Brain Res. 2014;210:31-58. doi: 10.1016/B978-0-444-63356-9.00002-9. Prog Brain Res. 2014. PMID: 24916288 Review.
-
The cerebellar Golgi cell and spatiotemporal organization of granular layer activity.Front Neural Circuits. 2013 May 17;7:93. doi: 10.3389/fncir.2013.00093. eCollection 2013. Front Neural Circuits. 2013. PMID: 23730271 Free PMC article. Review.
Cited by
-
Mesoscale simulations predict the role of synergistic cerebellar plasticity during classical eyeblink conditioning.PLoS Comput Biol. 2024 Apr 4;20(4):e1011277. doi: 10.1371/journal.pcbi.1011277. eCollection 2024 Apr. PLoS Comput Biol. 2024. PMID: 38574161 Free PMC article.
-
Cerebellar control over inter-regional excitatory/inhibitory dynamics discriminates execution from observation of an action.Cerebellum. 2025 Jun 16;24(4):115. doi: 10.1007/s12311-025-01863-6. Cerebellum. 2025. PMID: 40524122 Free PMC article.
-
Variations on the theme: focus on cerebellum and emotional processing.Front Syst Neurosci. 2023 May 10;17:1185752. doi: 10.3389/fnsys.2023.1185752. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37234065 Free PMC article. Review.
-
Region-specific mean field models enhance simulations of local and global brain dynamics.NPJ Syst Biol Appl. 2025 Jun 24;11(1):66. doi: 10.1038/s41540-025-00543-9. NPJ Syst Biol Appl. 2025. PMID: 40555720 Free PMC article.
-
Discovering Microcircuit Secrets With Multi-Spot Imaging and Electrophysiological Recordings: The Example of Cerebellar Network Dynamics.Front Cell Neurosci. 2022 Mar 18;16:805670. doi: 10.3389/fncel.2022.805670. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35370553 Free PMC article. Review.
References
-
- Arbib MA, Érdi P. Precis of neural organization: Structure, function, and dynamics. Behav. Brain Sci. 2000;23:513–533. - PubMed
-
- D’Angelo E, Gandini Wheeler-Kingshott C. Modelling the brain: Elementary components to explain ensemble functions. Riv. del. nuovo Cim. 2017;40:273–333.
-
- Markram H, et al. Reconstruction and Simulation of Neocortical Microcircuitry. Cell. 2015;163:456–492. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

