Surfactant protein D inhibits lipid-laden foamy macrophages and lung inflammation in chronic obstructive pulmonary disease
- PMID: 36376488
- PMCID: PMC9794778
- DOI: 10.1038/s41423-022-00946-2
Surfactant protein D inhibits lipid-laden foamy macrophages and lung inflammation in chronic obstructive pulmonary disease
Abstract
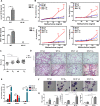
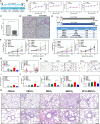
Increased levels of surfactant protein D (SP-D) and lipid-laden foamy macrophages (FMs) are frequently found under oxidative stress conditions and/or in patients with chronic obstructive pulmonary disease (COPD) who are also chronically exposed to cigarette smoke (CS). However, the roles and molecular mechanisms of SP-D and FMs in COPD have not yet been determined. In this study, increased levels of SP-D were found in the bronchoalveolar lavage fluid (BALF) and sera of ozone- and CS-exposed mice. Furthermore, SP-D-knockout mice showed increased lipid-laden FMs and airway inflammation caused by ozone and CS exposure, similar to that exhibited by our study cohort of chronic smokers and COPD patients. We also showed that an exogenous recombinant fragment of human SP-D (rfhSP-D) prevented the formation of oxidized low-density lipoprotein (oxLDL)-induced FMs in vitro and reversed the airway inflammation and emphysematous changes caused by oxidative stress and CS exposure in vivo. SP-D upregulated bone marrow-derived macrophage (BMDM) expression of genes involved in countering the oxidative stress and lipid metabolism perturbations induced by CS and oxLDL. Our study demonstrates the crucial roles of SP-D in the lipid homeostasis of dysfunctional alveolar macrophages caused by ozone and CS exposure in experimental mouse emphysema, which may provide a novel opportunity for the clinical application of SP-D in patients with COPD.
Keywords: Alveolar macrophages; Chronic obstructive pulmonary diseases; Cigarettes; Lipid metabolism; Ozone; Surfactant protein D.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

