Induction of Nanog in neural progenitor cells for adaptive regeneration of ischemic brain
- PMID: 36376495
- PMCID: PMC9722910
- DOI: 10.1038/s12276-022-00880-3
Induction of Nanog in neural progenitor cells for adaptive regeneration of ischemic brain
Abstract
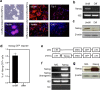
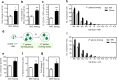
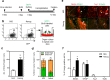
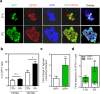
NANOG plays a key role in cellular plasticity and the acquisition of the stem cell state during reprogramming, but its role in the regenerative process remains unclear. Here, we show that the induction of NANOG in neuronal cells is necessary for the physiological initiation of neuronal regeneration in response to ischemic stress. Specifically, we found that NANOG was preferentially expressed in undifferentiated neuronal cells, and forced expression of Nanog in neural progenitor cells (NPCs) promoted their self-renewing expansion both in ex-vivo slice cultures and in vitro limiting dilution analysis. Notably, the upstream region of the Nanog gene contains sequence motifs for hypoxia-inducible factor-1 alpha (HIF-1α). Therefore, cerebral neurons exposed to hypoxia significantly upregulated NANOG expression selectively in primitive (CD133+) cells, but not in mature cells, leading to the expansion of NPCs. Notably, up to 80% of the neuronal expansion induced by hypoxia was attributed to NANOG-expressing neuronal cells, whereas knockdown during hypoxia abolished this expansion and was accompanied by the downregulation of other pluripotency-related genes. Moreover, the number of NANOG-expressing neuronal cells were transiently increased in response to ischemic insult, predominantly in the infarct area of brain regions undergoing neurogenesis, but not in non-neurogenic loci. Together, these findings reveal a functional effect of NANOG-induction for the initiation of adaptive neuronal regeneration among heterogeneous NPC subsets, pointing to cellular plasticity as a potential link between regeneration and reprogramming processes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Roles for HIF-1α in neural stem cell function and the regenerative response to stroke.Behav Brain Res. 2012 Feb 14;227(2):410-7. doi: 10.1016/j.bbr.2011.08.002. Epub 2011 Aug 18. Behav Brain Res. 2012. PMID: 21871501 Free PMC article. Review.
-
Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m⁶A-demethylation of NANOG mRNA.Proc Natl Acad Sci U S A. 2016 Apr 5;113(14):E2047-56. doi: 10.1073/pnas.1602883113. Epub 2016 Mar 21. Proc Natl Acad Sci U S A. 2016. PMID: 27001847 Free PMC article.
-
Alternative Routes to Induced Pluripotent Stem Cells Revealed by Reprogramming of the Neural Lineage.Stem Cell Reports. 2016 Mar 8;6(3):302-11. doi: 10.1016/j.stemcr.2016.01.009. Epub 2016 Feb 18. Stem Cell Reports. 2016. PMID: 26905202 Free PMC article.
-
The Pluripotency Factor Nanog Protects against Neuronal Amyloid β-Induced Toxicity and Oxidative Stress through Insulin Sensitivity Restoration.Cells. 2020 May 27;9(6):1339. doi: 10.3390/cells9061339. Cells. 2020. PMID: 32471175 Free PMC article.
-
From Vessels to Neurons-The Role of Hypoxia Pathway Proteins in Embryonic Neurogenesis.Cells. 2024 Apr 3;13(7):621. doi: 10.3390/cells13070621. Cells. 2024. PMID: 38607059 Free PMC article. Review.
Cited by
-
Influence of Hypoxic Condition on Cytotoxicity, Cellular Migration, and Osteogenic Differentiation Potential of Aged Periodontal Ligament Cells.Eur J Dent. 2025 Feb;19(1):70-79. doi: 10.1055/s-0044-1786844. Epub 2024 May 17. Eur J Dent. 2025. PMID: 38759996 Free PMC article.
-
The influence of biomimetic conditions on neurogenic and neuroprotective properties of dedifferentiated fat cells.Stem Cells. 2025 Jan 17;43(1):sxae066. doi: 10.1093/stmcls/sxae066. Stem Cells. 2025. PMID: 39576128 Free PMC article.
-
Leveraging chromatin state transitions for the identification of regulatory networks orchestrating heart regeneration.Nucleic Acids Res. 2024 May 8;52(8):4215-4233. doi: 10.1093/nar/gkae085. Nucleic Acids Res. 2024. PMID: 38364861 Free PMC article.
-
Hypoxia-induced cancer cell reprogramming: a review on how cancer stem cells arise.Front Oncol. 2023 Aug 8;13:1227884. doi: 10.3389/fonc.2023.1227884. eCollection 2023. Front Oncol. 2023. PMID: 37614497 Free PMC article. Review.
-
Cortical Organoid-on-a-Chip with Physiological Hypoxia for Investigating Tanshinone IIA-Induced Neural Differentiation.Research (Wash D C). 2023 Nov 22;6:0273. doi: 10.34133/research.0273. eCollection 2023. Research (Wash D C). 2023. PMID: 38434243 Free PMC article.
References
-
- Alvarez-Buylla A, Herrera DG, Wichterle H. The subventricular zone: source of neuronal precursors for brain repair. Prog. Brain Res. 2000;127:1–11. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. - PubMed
-
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. - PubMed
-
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr. Opin. Neurobiol. 2005;15:514–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

