Notch1 and Notch4 core binding domain peptibodies exhibit distinct ligand-binding and anti-angiogenic properties
- PMID: 36376768
- PMCID: PMC10119233
- DOI: 10.1007/s10456-022-09861-6
Notch1 and Notch4 core binding domain peptibodies exhibit distinct ligand-binding and anti-angiogenic properties
Abstract
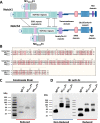
The Notch signaling pathway is an important therapeutic target for the treatment of inflammatory diseases and cancer. We previously created ligand-specific inhibitors of Notch signaling comprised of Fc fusions to specific EGF-like repeats of the Notch1 extracellular domain, called Notch decoys, which bound ligands, blocked Notch signaling, and showed anti-tumor activity with low toxicity. However, the study of their function depended on virally mediated expression, which precluded dosage control and limited clinical applicability. We have refined the decoy design to create peptibody-based Notch inhibitors comprising the core binding domains, EGF-like repeats 10-14, of either Notch1 or Notch4. These Notch peptibodies showed high secretion properties and production yields that were improved by nearly 100-fold compared to previous Notch decoys. Using surface plasmon resonance spectroscopy coupled with co-immunoprecipitation assays, we observed that Notch1 and Notch4 peptibodies demonstrate strong but distinct binding properties to Notch ligands DLL4 and JAG1. Both Notch1 and Notch4 peptibodies interfere with Notch signaling in endothelial cells and reduce expression of canonical Notch targets after treatment. While prior DLL4 inhibitors cause hyper-sprouting, the Notch1 peptibody reduced angiogenesis in a 3-dimensional in vitro sprouting assay. Administration of Notch1 peptibodies to neonate mice resulted in reduced radial outgrowth of retinal vasculature, confirming anti-angiogenic properties. We conclude that purified Notch peptibodies comprising EGF-like repeats 10-14 bind to both DLL4 and JAG1 ligands and exhibit anti-angiogenic properties. Based on their secretion profile, unique Notch inhibitory activities, and anti-angiogenic properties, Notch peptibodies present new opportunities for therapeutic Notch inhibition.
Keywords: Angiogenesis; Inhibitor; Notch; Notch core binding domain; Peptibody.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth.Cancer Discov. 2015 Feb;5(2):182-97. doi: 10.1158/2159-8290.CD-14-0650. Epub 2014 Nov 11. Cancer Discov. 2015. PMID: 25387766 Free PMC article.
-
Endothelial Jagged1 antagonizes Dll4 regulation of endothelial branching and promotes vascular maturation downstream of Dll4/Notch1.Arterioscler Thromb Vasc Biol. 2015 May;35(5):1134-46. doi: 10.1161/ATVBAHA.114.304741. Epub 2015 Mar 12. Arterioscler Thromb Vasc Biol. 2015. PMID: 25767274
-
Intraovarian regulation of gonadotropin-dependent folliculogenesis depends on notch receptor signaling pathways not involving Delta-like ligand 4 (Dll4).Reprod Biol Endocrinol. 2013 May 15;11:43. doi: 10.1186/1477-7827-11-43. Reprod Biol Endocrinol. 2013. PMID: 23675950 Free PMC article.
-
Notch functions in developmental and tumour angiogenesis by diverse mechanisms.Biochem Soc Trans. 2014 Dec;42(6):1563-8. doi: 10.1042/BST20140233. Biochem Soc Trans. 2014. PMID: 25399571 Review.
-
Peptibodies: Bridging the gap between peptides and antibodies.Int J Biol Macromol. 2024 Oct;278(Pt 2):134718. doi: 10.1016/j.ijbiomac.2024.134718. Epub 2024 Aug 12. Int J Biol Macromol. 2024. PMID: 39142490 Review.
Cited by
-
Inhibition of Notch4 Using Novel Neutralizing Antibodies Reduces Tumor Growth in Murine Cancer Models by Targeting the Tumor Endothelium.Cancer Res Commun. 2024 Jul 1;4(7):1881-1893. doi: 10.1158/2767-9764.CRC-24-0081. Cancer Res Commun. 2024. PMID: 38984877 Free PMC article.
-
Coagulation Factor XII Is an Antibacterial Protein That Acts Against Bacterial Infection via Its Heavy Chain.Int J Mol Sci. 2025 Jun 23;26(13):6009. doi: 10.3390/ijms26136009. Int J Mol Sci. 2025. PMID: 40649792 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

