Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis
- PMID: 36376937
- PMCID: PMC9661756
- DOI: 10.1186/s40035-022-00323-z
Gut microbiome, cognitive function and brain structure: a multi-omics integration analysis
Abstract
Background: Microbiome-gut-brain axis may be involved in the progression of age-related cognitive impairment and relevant brain structure changes, but evidence from large human cohorts is lacking. This study was aimed to investigate the associations of gut microbiome with cognitive impairment and brain structure based on multi-omics from three independent populations.
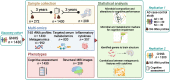
Methods: We included 1430 participants from the Guangzhou Nutrition and Health Study (GNHS) with both gut microbiome and cognitive assessment data available as a discovery cohort, of whom 272 individuals provided fecal samples twice before cognitive assessment. We selected 208 individuals with baseline microbiome data for brain magnetic resonance imaging during the follow-up visit. Fecal 16S rRNA and shotgun metagenomic sequencing, targeted serum metabolomics, and cytokine measurements were performed in the GNHS. The validation analyses were conducted in an Alzheimer's disease case-control study (replication study 1, n = 90) and another community-based cohort (replication study 2, n = 1300) with cross-sectional dataset.
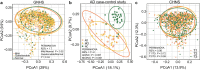
Results: We found protective associations of specific gut microbial genera (Odoribacter, Butyricimonas, and Bacteroides) with cognitive impairment in both the discovery cohort and the replication study 1. Result of Bacteroides was further validated in the replication study 2. Odoribacter was positively associated with hippocampal volume (β, 0.16; 95% CI 0.06-0.26, P = 0.002), which might be mediated by acetic acids. Increased intra-individual alterations in gut microbial composition were found in participants with cognitive impairment. We also identified several serum metabolites and inflammation-associated metagenomic species and pathways linked to impaired cognition.
Conclusions: Our findings reveal that specific gut microbial features are closely associated with cognitive impairment and decreased hippocampal volume, which may play an important role in dementia development.
Keywords: Brain structure; Cognitive impairment; Cohort; Gut microbiome; Metagenomics; Microbiome–gut–brain axis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Patterson C. World Alzheimer report 2018. London: Alzheimer’s Disease International; 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

