Neuroinflammation regulates the balance between hippocampal neuron death and neurogenesis in an ex vivo model of thiamine deficiency
- PMID: 36376954
- PMCID: PMC9664832
- DOI: 10.1186/s12974-022-02624-6
Neuroinflammation regulates the balance between hippocampal neuron death and neurogenesis in an ex vivo model of thiamine deficiency
Abstract
Background: Thiamine (vitamin B1) is a cofactor for enzymes of central energy metabolism and its deficiency (TD) impairs oxidative phosphorylation, increases oxidative stress, and activates inflammatory processes that can lead to neurodegeneration. Wernicke-Korsakoff syndrome (WKS) is a consequence of chronic TD, which leads to extensive neuronal death, and is associated with neuropathological disorders, including cognitive deficits and amnesia. The hippocampus is one of the brain areas most affected by WKS. B1 replacement may not be enough to prevent the irreversible cognitive deficit associated with WKS.
Materials and methods: An organotypic hippocampal slice culture (OHC) model was developed to investigate, using immunofluorescence and confocal microscopy and transcriptome analysis, the molecular mechanisms underlying the neurodegeneration associated with TD. The effect of anti-inflammatory pharmacological intervention with resveratrol (RSV) was also assessed in B1-deprived OHCs.
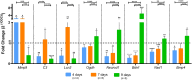
Results: In OHCs cultured without B1, neuronal density decayed after 5 days and, on the 7th day, the epigenetic markings H3K4me3 and H3K9me3 were altered in mature neurons likely favoring gene transcription. Between the 7th and the 14th day, a pulse of neurogenesis was observed followed by a further massive neuron loss. Transcriptome analysis at day nine disclosed 89 differentially expressed genes in response to B1 deprivation. Genes involved in tryptophan metabolism and lysine degradation KEGG pathways, and those with Gene Ontology (GO) annotations related to the organization of the extracellular matrix, cell adhesion, and positive regulation of synaptic transmission were upregulated. Several genes of the TNF and FoxO signaling pathways and with GO terms related to inflammation were inhibited in response to B1 deprivation. Nsd1, whose product methylates histone H3 lysine 36, was upregulated and the epigenetic marking H3K36me3, associated with negative regulation of neurogenesis, was increased in neurons. Treating B1-deprived OHCs with RSV promoted an earlier neurogenesis pulse.
Conclusion: Neuroregeneration occurs in B1-deficient hippocampal tissue during a time window. This phenomenon depends on reducing neuroinflammation and, likely, on metabolic changes, allowing acetyl-CoA synthesis from amino acids to ensure energy supply via oxidative phosphorylation. Thus, neuroinflammation is implicated as a major regulator of hippocampal neurogenesis in TD opening a new search space for treating WKS.
Keywords: Neurodegeneration; Neurogenesis. inflammation; Neuroregeneration; Organotypic hippocampal culture; Thiamine deficiency.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Nunes PT, Gómez-Mendoza DP, Rezende CP, Figueiredo HCP, Ribeiro AM. Thalamic proteome changes and behavioral impairments in thiamine-deficient rats. Neuroscience. 2018;385:181–197. - PubMed
-
- Bubber P, Ke ZJ, Gibson GE. Tricarboxylic acid cycle enzymes following thiamine deficiency. Neurochem Int. 2004;45(7):1021–1028. - PubMed
-
- Jhala SS, Hazell AS. Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int. 2011;58(3):248–260. - PubMed
-
- Inaba H, Kishimoto T, Oishi S, Nagata K, Hasegawa S, Watanabe T, et al. Vitamin B1-deficient mice show impairment of hippocampus-dependent memory formation and loss of hippocampal neurons and dendritic spines: potential microendophenotypes of Wernicke–Korsakoff syndrome. Biosci Biotechnol Biochem. 2016;80(12):2425–2436. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

