Dual-labeled nanoparticles based on small extracellular vesicles for tumor detection
- PMID: 36376978
- PMCID: PMC9664624
- DOI: 10.1186/s13062-022-00345-7
Dual-labeled nanoparticles based on small extracellular vesicles for tumor detection
Abstract
Background: Small extracellular vesicles (sEVs) are emerging natural nanoplatforms in cancer diagnosis and therapy, through the incorporation of signal components or drugs in their structure. However, for their translation into the clinical field, there is still a lack of tools that enable a deeper understanding of their in vivo pharmacokinetics or their interactions with the cells of the tumor microenvironment. In this study, we have designed a dual-sEV probe based on radioactive and fluorescent labeling of goat milk sEVs.
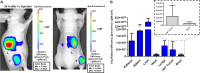
Results: The imaging nanoprobe was tested in vitro and in vivo in a model of glioblastoma. In vitro assessment of the uptake of the dual probe in different cell populations (RAW 264.7, U87, and HeLa) by optical and nuclear techniques (gamma counter, confocal imaging, and flow cytometry) revealed the highest uptake in inflammatory cells (RAW 264.7), followed by glioblastoma U87 cells. In vivo evaluation of the pharmacokinetic properties of nanoparticles confirmed a blood circulation time of ~ 8 h and primarily hepatobiliary elimination. The diagnostic capability of the dual nanoprobe was confirmed in vivo in a glioblastoma xenograft model, which showed intense in vivo uptake of the SEV-based probe in tumor tissue. Histological assessment by confocal imaging enabled quantification of tumor populations and confirmed uptake in tumor cells and tumor-associated macrophages, followed by cancer-associated fibroblasts and endothelial cells.
Conclusions: We have developed a chemical approach for dual radioactive and fluorescent labeling of sEVs. This methodology enables in vivo and in vitro study of these vesicles after exogenous administration. The dual nanoprobe would be a promising technology for cancer diagnosis and a powerful tool for studying the biological behavior of these nanosystems for use in drug delivery.
Keywords: Diagnosis; Extracellular vesicles; Molecular imaging; Oncology; Optical imaging; SPECT.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Liu M, Anderson R-C, Lan X, Conti PS, Chen K. Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer. Med Res Rev. 2020;40:909–930. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

