Importance of assessing biomarkers and physiological parameters of anemia-induced tissue hypoxia in the perioperative period
- PMID: 36377057
- PMCID: PMC10068554
- DOI: 10.1016/j.bjane.2022.10.004
Importance of assessing biomarkers and physiological parameters of anemia-induced tissue hypoxia in the perioperative period
Abstract
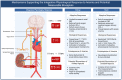
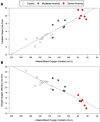
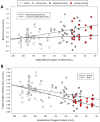
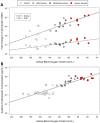
Anemia is associated with increased risk of Acute Kidney Injury (AKI), stroke and mortality in perioperative patients. We sought to understand the mechanism(s) by assessing the integrative physiological responses to anemia (kidney, brain), the degrees of anemia-induced tissue hypoxia, and associated biomarkers and physiological parameters. Experimental measurements demonstrate a linear relationship between blood Oxygen Content (CaO2) and renal microvascular PO2 (y = 0.30x + 6.9, r2 = 0.75), demonstrating that renal hypoxia is proportional to the degree of anemia. This defines the kidney as a potential oxygen sensor during anemia. Further evidence of renal oxygen sensing is demonstrated by proportional increase in serum Erythropoietin (EPO) during anemia (y = 93.806*10-0.02, r2 = 0.82). This data implicates systemic EPO levels as a biomarker of anemia-induced renal tissue hypoxia. By contrast, cerebral Oxygen Delivery (DO2) is defended by a profound proportional increase in Cerebral Blood Flow (CBF), minimizing tissue hypoxia in the brain, until more severe levels of anemia occur. We hypothesize that the kidney experiences profound early anemia-induced tissue hypoxia which contributes to adaptive mechanisms to preserve cerebral perfusion. At severe levels of anemia, renal hypoxia intensifies, and cerebral hypoxia occurs, possibly contributing to the mechanism(s) of AKI and stroke when adaptive mechanisms to preserve organ perfusion are overwhelmed. Clinical methods to detect renal tissue hypoxia (an early warning signal) and cerebral hypoxia (a later consequence of severe anemia) may inform clinical practice and support the assessment of clinical biomarkers (i.e., EPO) and physiological parameters (i.e., urinary PO2) of anemia-induced tissue hypoxia. This information may direct targeted treatment strategies to prevent adverse outcomes associated with anemia.
Keywords: Anemia; Brain; Erythropoietin; Hypoxia; Kidney; Perioperative period.
Copyright © 2022. Published by Elsevier España, S.L.U.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures

References
-
- Goobie SM, DiNardo JA, Faraoni D. Relationship between transfusion volume and outcomes in children undergoing noncardiac surgery. Transfusion. 2016;56:2487–2494. - PubMed
-
- Daru J, Zamora J, Fernández-Félix BM, et al. Risk of maternal mortality in women with severe anaemia during pregnancy and post partum: a multilevel analysis. Lancet Glob Health. 2018;6:e548–e554. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

