Experimental and In Silico Analysis of TEM β-Lactamase Adaptive Evolution
- PMID: 36377311
- PMCID: PMC9745794
- DOI: 10.1021/acsinfecdis.2c00216
Experimental and In Silico Analysis of TEM β-Lactamase Adaptive Evolution
Abstract
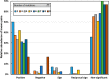
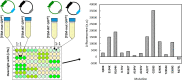
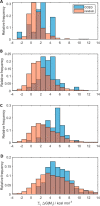
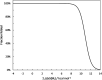
Multiple mutations often have non-additive (epistatic) phenotypic effects. Epistasis is of fundamental biological relevance but is not well understood mechanistically. Adaptive evolution, i.e., the evolution of new biochemical activities, is rich in epistatic interactions. To better understand the principles underlying epistasis during genetic adaptation, we studied the evolution of TEM-1 β-lactamase variants exhibiting cefotaxime resistance. We report the collection of a library of 487 observed evolutionary trajectories for TEM-1 and determine the epistasis status based on cefotaxime resistance phenotype for 206 combinations of 2-3 TEM-1 mutations involving 17 positions under adaptive selective pressure. Gain-of-function (GOF) mutations are gatekeepers for adaptation. To see if GOF phenotypes can be inferred based solely on sequence data, we calculated the enrichment of GOF mutations in the different categories of epistatic pairs. Our results suggest that this is possible because GOF mutations are particularly enriched in sign and reciprocal sign epistasis, which leave a major imprint on the sequence space accessible to evolution. We also used FoldX to explore the relationship between thermodynamic stability and epistasis. We found that mutations in observed evolutionary trajectories tend to destabilize the folded structure of the protein, albeit their cumulative effects are consistently below the protein's free energy of folding. The destabilizing effect is stronger for epistatic pairs, suggesting that modest or local alterations in folding stability can modulate catalysis. Finally, we report a significant relationship between epistasis and the degree to which two protein positions are structurally and dynamically coupled, even in the absence of ligand.
Keywords: antibiotic resistance; contingency; epistasis; evolution; fitness landscape; selection.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
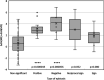
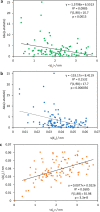
References
-
- Hartman E. C.; Tullman-Ercek D. Learning from Protein Fitness Landscapes: A Review of Mutability, Epistasis, and Evolution. Curr. Opin. Syst. Biol. 2019, 14, 25–31. 10.1016/j.coisb.2019.02.006. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

